LC-MS-based metabolomics in drug metabolism
- PMID: 17786640
- PMCID: PMC2140249
- DOI: 10.1080/03602530701497804
LC-MS-based metabolomics in drug metabolism
Abstract
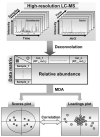
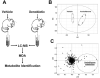
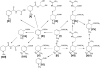
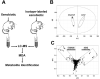
Xenobiotic metabolism, a ubiquitous natural response to foreign compounds, elicits initiating signals for many pathophysiological events. Currently, most widely used techniques for identifying xenobiotic metabolites and metabolic pathways are empirical and largely based on in vitro incubation assays and in vivo radiotracing experiments. Recent work in our lab has shown that LC-MS-based metabolomic techniques are useful tools for xenobiotic metabolism research since multivariate data analysis in metabolomics can significantly rationalize the processes of xenobiotic metabolite identification and metabolic pathway analysis. In this review, the technological elements of LC-MS-based metabolomics for constructing high-quality datasets and conducting comprehensive data analysis are examined. Four novel approaches of using LC-MS-based metabolomic techniques in xenobiotic metabolism research are proposed and illustrated by case studies and proof-of-concept experiments, and the perspective on their application is further discussed.
Figures

References
-
- Allen JG, Brown AN, Marten TR. Metabolism of debrisoquine sulphate in rat, dog and man. Xenobiotica. 1976;6:405–409. - PubMed
-
- Allen JG, East PB, Francis RJ, Haigh JL. Metabolism of debrisoquine sulfate. Identification of some urinary metabolites in rat and man. Drug Metab. Dispos. 1975;3:332–337. - PubMed
-
- Ando Y, Saka H, Ando M, Sawa T, Muro K, Ueoka H, Yokoyama A, Saitoh S, Shimokata K, Hasegawa Y. Polymorphisms of UDP-glucuronosyltransferase gene and irinotecan toxicity: a pharmacogenetic analysis. Cancer Res. 2000;60:6921–6926. - PubMed
-
- Brindle JT, Antti H, Holmes E, Tranter G, Nicholson JK, Bethell HW, Clarke S, Schofield PM, McKilligin E, Mosedale DE, Grainger DJ. Rapid and noninvasive diagnosis of the presence and severity of coronary heart disease using 1H-NMR-based metabonomics. Nat. Med. 2002;8:1439–1444. - PubMed
-
- Brodie BB, Axelrod J. The Fate of Acetanilide in Man. J. Pharmacol. Exp. Ther. 1948;94:29. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
