Evaluation of the efficacy and safety of ramelteon in subjects with chronic insomnia
- PMID: 17803013
- PMCID: PMC1978328
Evaluation of the efficacy and safety of ramelteon in subjects with chronic insomnia
Erratum in
- J Clin Sleep Med. 2007 Oct 15;3(6):table of contents
- J Clin Sleep Med. 2008 Oct 15;4(5):table of contents
Abstract
Objective: To evaluate efficacy and safety of ramelteon (MT1/MT2-receptor [corrected] agonist) in subjects with chronic primary insomnia.
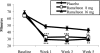
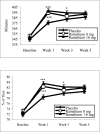
Methods: Randomized, multicenter, double-blind, placebo-controlled trial of nightly ramelteon treatment (8 mg or 16 mg) in adults (N=405) with primary chronic insomnia (DSM-IV-TR). Latency to persistent sleep (LPS), TST, sleep efficiency, wake time after sleep onset, and number of awakenings were measured by polysomnography. Subject-reported measures were also assessed.
Results: LPS at Week 1 (primary measure) was significantly shorter with ramelteon 8 mg (32.2 min) or 16 mg (28.9 min) vs placebo (47.9 min; p <0.001). Significant improvements in LPS were maintained at Weeks 3 and 5. TST was significantly longer with both doses of ramelteon at Week 1 (p <0.001) vs placebo. Subject-reported sleep latency was significantly shorter with ramelteon 8 mg at Weeks 1, 3, and 5 (p <0.001) and ramelteon 16 mg at Weeks 1 and 3 (p < or =0.050) vs placebo. Wake time after sleep onset and number of awakenings were not significantly different with ramelteon 8 mg or 16 mg treatment vs placebo. Subjective TST was significantly longer with ramelteon 8 mg at Weeks 1, 3, and 5 (p < or =0.050) and ramelteon 16 mg at Week 1 (p = 0.003) vs placebo. Ramelteon had no clinically meaningful effect on sleep architecture, next-morning psychomotor tasks, alertness, or ability to concentrate. No withdrawal or rebound effects were observed.
Conclusions: Ramelteon reduced LPS over 5 weeks of treatment in subjects with chronic insomnia, with no clinically meaningful sleep architecture alterations, next-morning residual pharmacologic effects, and no evidence of rebound insomnia or withdrawal. No numerical differences were observed between the 2 doses of ramelteon.
Figures

Similar articles
-
Efficacy and safety of 6-month nightly ramelteon administration in adults with chronic primary insomnia.Sleep. 2009 Mar;32(3):351-60. doi: 10.1093/sleep/32.3.351. Sleep. 2009. PMID: 19294955 Free PMC article. Clinical Trial.
-
Effects of ramelteon on patient-reported sleep latency in older adults with chronic insomnia.Sleep Med. 2006 Jun;7(4):312-8. doi: 10.1016/j.sleep.2006.01.003. Epub 2006 May 18. Sleep Med. 2006. PMID: 16709464 Clinical Trial.
-
A 2-night, 3-period, crossover study of ramelteon's efficacy and safety in older adults with chronic insomnia.Curr Med Res Opin. 2007 May;23(5):1005-14. doi: 10.1185/030079907x178874. Curr Med Res Opin. 2007. PMID: 17519067 Clinical Trial.
-
Ramelteon for the treatment of insomnia.Clin Ther. 2006 Oct;28(10):1540-55. doi: 10.1016/j.clinthera.2006.10.016. Clin Ther. 2006. PMID: 17157111 Review.
-
Ramelteon: a review of its use in insomnia.Drugs. 2008;68(13):1901-19. doi: 10.2165/00003495-200868130-00011. Drugs. 2008. PMID: 18729542 Review.
Cited by
-
Mood disorders, circadian rhythms, melatonin and melatonin agonists.J Cent Nerv Syst Dis. 2012 Jan 4;4:15-26. doi: 10.4137/JCNSD.S4103. Print 2012. J Cent Nerv Syst Dis. 2012. PMID: 23650464 Free PMC article.
-
Effect of ramelteon, a selective MT(1)/MT (2)-receptor agonist, on respiration during sleep in mild to moderate COPD.Sleep Breath. 2008 Aug;12(3):243-50. doi: 10.1007/s11325-007-0156-4. Sleep Breath. 2008. PMID: 18060441 Clinical Trial.
-
The effects of ramelteon on respiration during sleep in subjects with moderate to severe chronic obstructive pulmonary disease.Sleep Breath. 2009 Mar;13(1):79-84. doi: 10.1007/s11325-008-0196-4. Epub 2008 Jun 27. Sleep Breath. 2009. PMID: 18584227 Clinical Trial.
-
New approaches in the management of insomnia: weighing the advantages of prolonged-release melatonin and synthetic melatoninergic agonists.Neuropsychiatr Dis Treat. 2009;5:341-54. doi: 10.2147/ndt.s4234. Epub 2009 Jun 10. Neuropsychiatr Dis Treat. 2009. PMID: 19557144 Free PMC article.
-
Comorbid insomnia in sleep-related breathing disorders: an under-recognized association.Sleep Breath. 2012 Jun;16(2):295-304. doi: 10.1007/s11325-011-0513-1. Epub 2011 Mar 29. Sleep Breath. 2012. PMID: 21445659 Review.
References
-
- Roth T, Roehrs T. Insomnia: epidemiology, characteristics, and consequences. Clin Cornerstone. 2003;5:5–15. - PubMed
-
- Shochat T, Umphress J, Israel AG, Ancoli-Israel S. Insomnia in primary care patients. Sleep. 1999;22:S359–65. - PubMed
-
- Allain H, Bentue-Ferrer D, Tarral A, Gandon J. Effects on postural oscillation and memory functions of a single dose of zolpidem 5 mg, zopiclone 3.75 mg and lormetazepam 1 mg in elderly healthy subjects. A randomized, cross-over, double-blind study versus placebo. Eur J Clin Pharmacol. 2003;59:179–88. - PubMed
-
- Roehrs T, Merlotti L, Zorick F, Roth T. Sedative, memory, and performance effects of hypnotics. Psychopharmacology (Berl) 1994;116:130–4. - PubMed
-
- Mintzer MZ, Frey JM, Yingling JE, Griffiths RR. Triazolam and zolpidem: a comparison of their psychomotor, cognitive, and subjective effects in healthy volunteers. Behav Pharmacol. 1997;8:561–74. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
