Human papillomavirus E6 regulates the cytoskeleton dynamics of keratinocytes through targeted degradation of p53
- PMID: 17804489
- PMCID: PMC2168984
- DOI: 10.1128/JVI.01083-07
Human papillomavirus E6 regulates the cytoskeleton dynamics of keratinocytes through targeted degradation of p53
Abstract
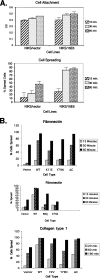
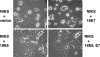
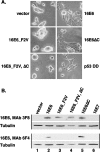
The attachment and spreading of keratinocyte cells result from interactions between integrins and immobilized extracellular matrix molecules. Human papillomavirus type 16 (HPV-16) E6 augmented the kinetics of cell spreading, while E6 genes from HPV-11 or bovine papillomavirus type 1 did not. The ability of E6 to interact with the E6AP ubiquitin ligase and target p53 degradation was required to augment cell-spreading kinetics; dominant negative p53 alleles also enhanced the kinetics of cell spreading and the level of attachment of cells to hydrophobic surfaces. The targeted degradation of p53 by E6 may contribute to the invasive phenotype exhibited by cervical cells that contain high-risk HPV types.
Figures

Similar articles
-
Multiple regions of E6AP (UBE3A) contribute to interaction with papillomavirus E6 proteins and the activation of ubiquitin ligase activity.PLoS Pathog. 2020 Jan 23;16(1):e1008295. doi: 10.1371/journal.ppat.1008295. eCollection 2020 Jan. PLoS Pathog. 2020. PMID: 31971989 Free PMC article.
-
Structure of the E6/E6AP/p53 complex required for HPV-mediated degradation of p53.Nature. 2016 Jan 28;529(7587):541-5. doi: 10.1038/nature16481. Epub 2016 Jan 20. Nature. 2016. PMID: 26789255 Free PMC article.
-
Regulation of human papillomavirus E6 oncoprotein function via a novel ubiquitin ligase FBXO4.mBio. 2025 Feb 5;16(2):e0278324. doi: 10.1128/mbio.02783-24. Epub 2024 Dec 17. mBio. 2025. PMID: 39688415 Free PMC article.
-
The role of TP53 in Cervical carcinogenesis.Hum Mutat. 2003 Mar;21(3):307-12. doi: 10.1002/humu.10178. Hum Mutat. 2003. PMID: 12619117 Review.
-
HPV E6, E6AP and cervical cancer.BMC Biochem. 2008 Oct 21;9 Suppl 1(Suppl 1):S4. doi: 10.1186/1471-2091-9-S1-S4. BMC Biochem. 2008. PMID: 19007434 Free PMC article. Review.
Cited by
-
Human papillomavirus type 16 E6 induces cell competition.PLoS Pathog. 2022 Mar 23;18(3):e1010431. doi: 10.1371/journal.ppat.1010431. eCollection 2022 Mar. PLoS Pathog. 2022. PMID: 35320322 Free PMC article.
-
Cellular binding partners of the human papillomavirus E6 protein.Arch Virol. 2008;153(3):397-408. doi: 10.1007/s00705-007-0022-5. Epub 2008 Jan 3. Arch Virol. 2008. PMID: 18172569 Free PMC article. Review.
-
Human papillomavirus E6 and E7 oncoproteins as risk factors for tumorigenesis.J Biosci. 2009 Mar;34(1):113-23. doi: 10.1007/s12038-009-0013-7. J Biosci. 2009. PMID: 19430123 Review.
-
Papillomavirus E6 PDZ interactions can be replaced by repression of p53 to promote episomal human papillomavirus genome maintenance.J Virol. 2014 Mar;88(5):3027-30. doi: 10.1128/JVI.02360-13. Epub 2013 Dec 18. J Virol. 2014. PMID: 24352452 Free PMC article.
-
Expression of E6, p53 and p21 proteins and physical state of HPV16 in cervical cytologies with and without low grade lesions.Int J Clin Exp Med. 2014 Jan 15;7(1):186-93. eCollection 2014. Int J Clin Exp Med. 2014. PMID: 24482706 Free PMC article.
References
-
- Allen-Hoffmann, B. L., S. J. Schlosser, C. A. Ivarie, C. A. Sattler, L. F. Meisner, and S. L. O'Connor. 2000. Normal growth and differentiation in a spontaneously immortalized near-diploid human keratinocyte cell line, NIKS. J. Investig. Dermatol. 114:444-455. - PubMed
-
- Cooper, B., S. Schneider, J. Bohl, Y. Jiang, A. Beaudet, and S. Vande Pol. 2003. Requirement of E6AP and the features of human papillomavirus E6 necessary to support degradation of p53. Virology 306:87-99. - PubMed
-
- Grm, H. S., and L. Banks. 2004. Degradation of hDlg and MAGIs by human papillomavirus E6 is E6-AP-independent. J. Gen. Virol. 85:2815-2819. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

