Claudin-6 and claudin-9 function as additional coreceptors for hepatitis C virus
- PMID: 17804490
- PMCID: PMC2169001
- DOI: 10.1128/JVI.01457-07
Claudin-6 and claudin-9 function as additional coreceptors for hepatitis C virus
Abstract
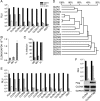
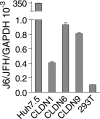
Hepatitis C virus (HCV) is a global challenge to public health. Several factors have been proven to be critical for HCV entry, including the newly identified claudin-1 (CLDN1). However, the mechanism of HCV entry is still obscure. Presently, among the 20 members of the claudin family identified in humans so far, CLDN1 has been the only member shown to be necessary for HCV entry. Recently, we discovered that Bel7402, an HCV-permissive cell line, does not express CLDN1 but expresses other members of claudin family. Among these claudins, CLDN9 was able to mediate HCV entry just as efficiently as CLDN1. We then examined if other members of the claudin family could mediate entry. We show that CLDN6 and CLDN9, but not CLDN2, CLDN3, CLDN4, CLDN7, CLDN11, CLDN12, CLDN15, CLDN17, and CLDN23, were able to mediate the entry of HCV into target cells. We found that CLDN6 and CLDN9 are expressed in the liver, the primary site of HCV replication. We also showed that CLDN6 and CLDN9, but not CLDN1, are expressed in peripheral blood mononuclear cells, an additional site of HCV replication. Through sequence comparison and mutagenesis studies, we show that residues N38 and V45 in the first extracellular loop (EL1) of CLDN9 are necessary for HCV entry.
Figures
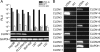
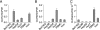

References
-
- Amoroso, P., M. Rapicetta, M. E. Tosti, A. Mele, E. Spada, S. Buonocore, G. Lettieri, P. Pierri, P. Chionne, A. R. Ciccaglione, and L. Sagliocca. 1998. Correlation between virus genotype and chronicity rate in acute hepatitis C. J. Hepatol. 28:939-944. - PubMed
-
- Bartosch, B., G. Verney, M. Dreux, P. Donot, Y. Morice, F. Penin, J. M. Pawlotsky, D. Lavillette, and F. L. Cosset. 2005. An interplay between hypervariable region 1 of the hepatitis C virus E2 glycoprotein, the scavenger receptor BI, and high-density lipoprotein promotes both enhancement of infection and protection against neutralizing antibodies. J. Virol. 79:8217-8229. - PMC - PubMed
-
- Blackard, J. T., N. Kemmer, and K. E. Sherman. 2006. Extrahepatic replication of HCV: insights into clinical manifestations and biological consequences. Hepatology 44:15-22. - PubMed
-
- Chen, R. M., D. H. Zhu, and X. Z. Ye. 1975. The establishment and character of human hepacarcinoma cell line (BEL-7402) in vitro. Comm. Sci. 20:434-436.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

