Interaction of decay-accelerating factor with coxsackievirus B3
- PMID: 17804498
- PMCID: PMC2169128
- DOI: 10.1128/JVI.00931-07
Interaction of decay-accelerating factor with coxsackievirus B3
Abstract
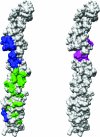
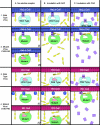
Many entero-, parecho-, and rhinoviruses use immunoglobulin (Ig)-like receptors that bind into the viral canyon and are required to initiate viral uncoating during infection. However, some of these viruses use an alternative or additional receptor that binds outside the canyon. Both the coxsackievirus-adenovirus receptor (CAR), an Ig-like molecule that binds into the viral canyon, and decay-accelerating factor (DAF) have been identified as cellular receptors for coxsackievirus B3 (CVB3). A cryoelectron microscopy reconstruction of a variant of CVB3 complexed with DAF shows full occupancy of the DAF receptor in each of 60 binding sites. The DAF molecule bridges the canyon, blocking the CAR binding site and causing the two receptors to compete with one another. The binding site of DAF on CVB3 differs from the binding site of DAF on the surface of echoviruses, suggesting independent evolutionary processes.
Figures






References
-
- Bhella, D., I. G. Goodfellow, P. Roversi, Y. Chaudhry, D. J. Evans, and S. M. Lea. 2004. The structure of echovirus type 12 bound to a two-domain fragment of its cellular attachment protein decay-accelerating factor (CD55). J. Biol. Chem. 279:8325-8332. - PubMed
-
- Brodbeck, W. G., D. Liu, J. Sperry, C. Mold, and M. E. Medof. 1996. Localization of classical and alternative pathway regulatory activity within the decay-accelerating factor. J. Immunol. 156:2528-2533. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

