Interactions between human immunodeficiency virus type 1 and vaccinia virus in human lymphoid tissue ex vivo
- PMID: 17804502
- PMCID: PMC2169030
- DOI: 10.1128/JVI.00326-07
Interactions between human immunodeficiency virus type 1 and vaccinia virus in human lymphoid tissue ex vivo
Abstract
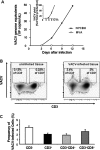
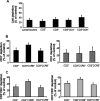
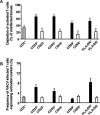
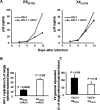
Vaccinia virus (VACV) has been attracting attention recently not only as a vector for various vaccines but also as an immunization tool against smallpox because of its potential use as a bioterrorism agent. It has become evident that in spite of a long history of studies of VACV, its tissue pathogenesis remains to be fully understood. Here, we investigated the pathogenesis of VACV and its interactions with human immunodeficiency virus type 1 (HIV-1) in the context of human lymphoid tissues. We found that ex vivo-cultured tonsillar tissue supports productive infection by the New York City Board of Health strain, the VACV strain of the Dryvax vaccine. VACV readily infected both T and non-T (B) lymphocytes and depleted cells of both of these subsets equally over a 12-day period postinfection. Among T lymphocytes, CD8(+) cells are preferentially depleted in accordance with their preferential infection: the probability that a CD8(+) T cell will be productively infected is almost six times higher than for a CD4(+) T cell. T cells expressing CCR5 and the activation markers CD25, CD38, and HLA-DR are other major targets for infection by VACV in lymphoid tissue. As a consequence, VACV predominantly inhibits the replication of the R5(SF162) phenotype of HIV-1 in coinfected tissues, as R5-tropic HIV-1 requires activated CCR5(+) CD4(+) cells for productive infection. Human lymphoid tissue infected ex vivo by VACV can be used to investigate interactions of VACV with other viruses, in particular HIV-1, and to evaluate various VACV vectors for the purpose of recombinant vaccine development.
Figures
Similar articles
-
Viral interactions in human lymphoid tissue: Human herpesvirus 7 suppresses the replication of CCR5-tropic human immunodeficiency virus type 1 via CD4 modulation.J Virol. 2007 Jan;81(2):708-17. doi: 10.1128/JVI.01367-06. Epub 2006 Oct 25. J Virol. 2007. PMID: 17065205 Free PMC article.
-
HIV-1 pathogenesis differs in rectosigmoid and tonsillar tissues infected ex vivo with CCR5- and CXCR4-tropic HIV-1.AIDS. 2007 Jun 19;21(10):1263-72. doi: 10.1097/QAD.0b013e3281864667. AIDS. 2007. PMID: 17545702
-
Segregation of R5 and X4 HIV-1 variants to memory T cell subsets differentially expressing CD62L in ex vivo infected human lymphoid tissue.AIDS. 2002 Jun 14;16(9):1245-9. doi: 10.1097/00002030-200206140-00006. AIDS. 2002. PMID: 12045489
-
Role of lymphoid organs in the pathogenesis of human immunodeficiency virus (HIV) infection.Immunol Rev. 1994 Aug;140:105-30. doi: 10.1111/j.1600-065x.1994.tb00867.x. Immunol Rev. 1994. PMID: 7821924 Review.
-
Uncovering the interplay between CD8, CD4 and antibody responses to complex pathogens.Future Microbiol. 2010 Feb;5(2):221-39. doi: 10.2217/fmb.09.110. Future Microbiol. 2010. PMID: 20143946 Free PMC article. Review.
Cited by
-
Histocultures (tissue explants) in human retrovirology.Methods Mol Biol. 2014;1087:233-48. doi: 10.1007/978-1-62703-670-2_19. Methods Mol Biol. 2014. PMID: 24158827 Free PMC article.
-
Use of human tissue explants to study human infectious agents.Nat Protoc. 2009;4(2):256-69. doi: 10.1038/nprot.2008.245. Nat Protoc. 2009. PMID: 19197269 Free PMC article.
-
Orbivirus NS4 Proteins Play Multiple Roles to Dampen Cellular Responses.Viruses. 2023 Sep 12;15(9):1908. doi: 10.3390/v15091908. Viruses. 2023. PMID: 37766314 Free PMC article.
-
Toxoplasma gondii inhibits R5 HIV-1 replication in human lymphoid tissues ex vivo.Microbes Infect. 2009 Dec;11(14-15):1106-13. doi: 10.1016/j.micinf.2009.08.004. Epub 2009 Aug 9. Microbes Infect. 2009. PMID: 19671446 Free PMC article.
-
Interactions between viral and prokaryotic pathogens in a mixed infection with cardiovirus and mycoplasma.J Virol. 2009 Oct;83(19):9940-51. doi: 10.1128/JVI.01167-09. Epub 2009 Jul 15. J Virol. 2009. PMID: 19605479 Free PMC article.
References
-
- Alonso, J. M., J. Rodriguez, E. Vinuela, G. Kroemer, and C. Martinez. 1991. Highly efficient expression of proteins encoded by recombinant vaccinia virus in lymphocytes. Scand. J. Immunol. 34:619-626. - PubMed
-
- Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H. L. Ma, B. D. Grimm, M. L. Hulsey, J. Miller, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 292:69-74. - PubMed
-
- Breman, J. G., and D. A. Henderson. 2002. Diagnosis and management of smallpox. N. Engl. J. Med. 346:1300-1308. - PubMed
-
- Bursill, C. A., J. L. Cash, K. M. Channon, and D. R. Greaves. 2006. Membrane-bound CC chemokine inhibitor 35K provides localized inhibition of CC chemokine activity in vitro and in vivo. J. Immunol. 177:5567-5573. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

