Skin-derived precursors generate myelinating Schwann cells that promote remyelination and functional recovery after contusion spinal cord injury
- PMID: 17804616
- PMCID: PMC6672973
- DOI: 10.1523/JNEUROSCI.1930-07.2007
Skin-derived precursors generate myelinating Schwann cells that promote remyelination and functional recovery after contusion spinal cord injury
Abstract
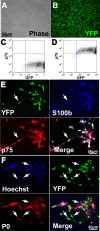
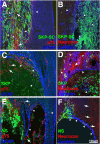
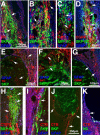
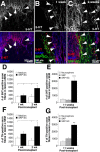
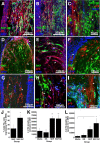
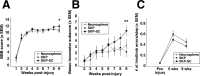
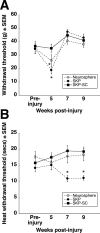
Transplantation of exogenous cells is one approach to spinal cord repair that could potentially enhance the growth and myelination of endogenous axons. Here, we asked whether skin-derived precursors (SKPs), a neural crest-like precursor that can be isolated and expanded from mammalian skin, could be used to repair the injured rat spinal cord. To ask this question, we isolated and expanded genetically tagged murine SKPs and either transplanted them directly into the contused rat spinal cord or differentiated them into Schwann cells (SCs), and performed similar transplantations with the isolated, expanded SKP-derived SCs. Neuroanatomical analysis of these transplants 12 weeks after transplantation revealed that both cell types survived well within the injured spinal cord, reduced the size of the contusion cavity, myelinated endogenous host axons, and recruited endogenous SCs into the injured cord. However, SKP-derived SCs also provided a bridge across the lesion site, increased the size of the spared tissue rim, myelinated spared axons within the tissue rim, reduced reactive gliosis, and provided an environment that was highly conducive to axonal growth. Importantly, SKP-derived SCs provided enhanced locomotor recovery relative to both SKPs and forebrain subventricular zone neurospheres, and had no impact on mechanical or heat sensitivity thresholds. Thus, SKP-derived SCs provide an accessible, potentially autologous source of cells for transplantation into and treatment of the injured spinal cord.
Figures


References
-
- Azanchi R, Bernal G, Gupta R, Keirstead HS. Combined demyelination plus Schwann cell transplantation therapy increases spread of cells and axonal regeneration following contusion injury. J Neurotrauma. 2004;21:775–788. - PubMed
-
- Basso DM. Behavioral testing after spinal cord injury: congruities, complexities, and controversies. J Neurotrauma. 2004;21:395–404. - PubMed
-
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. - PubMed
-
- Biernaskie J, McKenzie IA, Toma JG, Miller FD. Isolation of skin-derived precursors (SKPs) and differentiation and enrichment of their Schwann cell progeny. Nat Protoc. 2006;1:2803–2812. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
