Fate-mapping the mammalian hindbrain: segmental origins of vestibular projection neurons assessed using rhombomere-specific Hoxa2 enhancer elements in the mouse embryo
- PMID: 17804628
- PMCID: PMC6672974
- DOI: 10.1523/JNEUROSCI.2189-07.2007
Fate-mapping the mammalian hindbrain: segmental origins of vestibular projection neurons assessed using rhombomere-specific Hoxa2 enhancer elements in the mouse embryo
Abstract
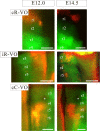
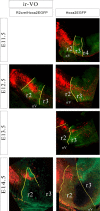
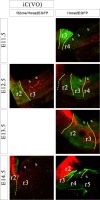
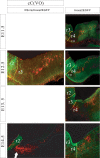
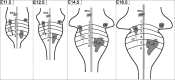
As a step toward generating a fate map of identified neuron populations in the mammalian hindbrain, we assessed the contributions of individual rhombomeres to the vestibular nuclear complex, a major sensorimotor area that spans the entire rhombencephalon. Transgenic mice harboring either the lacZ or the enhanced green fluorescent protein reporter genes under the transcriptional control of rhombomere-specific Hoxa2 enhancer elements were used to visualize rhombomere-derived domains. We labeled functionally identifiable vestibular projection neuron groups retrogradely with conjugated dextran-amines at successive embryonic stages and obtained developmental fate maps through direct comparison with the rhombomere-derived domains in the same embryos. The fate maps show that each vestibular neuron group derives from a unique rostrocaudal domain that is relatively stable developmentally, suggesting that anteroposterior migration is not a major contributor to the rostrocaudal patterning of the vestibular system. Most of the groups are multisegmental in origin, and each rhombomere is fated to give rise to two or more vestibular projection neuron types, in a complex pattern that is not segmentally iterated. Comparison with studies in the chicken embryo shows that the rostrocaudal patterning of identified vestibular projection neuron groups is generally well conserved between avians and mammalians but that significant species-specific differences exist in the rostrocaudal limits of particular groups. This mammalian hindbrain fate map can be used as the basis for targeting genetic manipulation to specific subpopulations of vestibular projection neurons.
Figures





References
-
- Altman J, Bayer SA. Development of the brain stem in the rat. III. Thymidine-radiographic study of the time of origin of neurons of the vestibular and auditory nuclei of the upper medulla. J Comp Neurol. 1980;194:877–904. - PubMed
-
- Anderson SA, Marin O, Horn C, Jennings K, Rubenstein JL. Distinct cortical migrations from the medial and lateral ganglionic eminences. Development. 2001;128:353–363. - PubMed
-
- Auclair F, Marchand R, Glover JC. Regional patterning of reticulospinal and vestibulospinal neurons in the hindbrain of rat and mouse embryos. J Comp Neurol. 1999;411:288–300. - PubMed
-
- Baader SL, Schilling ML, Rosengarten B, Pretsch W, Teutsch HF, Oberdick J, Schilling K. Purkinje cell lineage and the topographic organization of the cerebellar cortex: a view from X inactivation mosaics. Dev Biol. 1996;174:393–406. - PubMed
-
- Baker R, Highstein SM. Physiological identification of interneurons and motoneurons in the abducens nucleus. Brain Res. 1975;91:292–298. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
