Yield of stool culture with isolate toxin testing versus a two-step algorithm including stool toxin testing for detection of toxigenic Clostridium difficile
- PMID: 17804652
- PMCID: PMC2168505
- DOI: 10.1128/JCM.01305-07
Yield of stool culture with isolate toxin testing versus a two-step algorithm including stool toxin testing for detection of toxigenic Clostridium difficile
Abstract
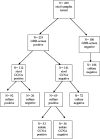
We examined the incremental yield of stool culture (with toxin testing on isolates) versus our two-step algorithm for optimal detection of toxigenic Clostridium difficile. Per the two-step algorithm, stools were screened for C. difficile-associated glutamate dehydrogenase (GDH) antigen and, if positive, tested for toxin by a direct (stool) cell culture cytotoxicity neutralization assay (CCNA). In parallel, stools were cultured for C. difficile and tested for toxin by both indirect (isolate) CCNA and conventional PCR if the direct CCNA was negative. The "gold standard" for toxigenic C. difficile was detection of C. difficile by the GDH screen or by culture and toxin production by direct or indirect CCNA. We tested 439 specimens from 439 patients. GDH screening detected all culture-positive specimens. The sensitivity of the two-step algorithm was 77% (95% confidence interval [CI], 70 to 84%), and that of culture was 87% (95% CI, 80 to 92%). PCR results correlated completely with those of CCNA testing on isolates (29/29 positive and 32/32 negative, respectively). We conclude that GDH is an excellent screening test and that culture with isolate CCNA testing detects an additional 23% of toxigenic C. difficile missed by direct CCNA. Since culture is tedious and also detects nontoxigenic C. difficile, we conclude that culture is most useful (i) when the direct CCNA is negative but a high clinical suspicion of toxigenic C. difficile remains, (ii) in the evaluation of new diagnostic tests for toxigenic C. difficile (where the best reference standard is essential), and (iii) in epidemiologic studies (where the availability of an isolate allows for strain typing and antimicrobial susceptibility testing).
Figures
References
-
- Aldeen, W. E., M. Bingham, A. Aiderzada, J. Kucera, S. Jense, and K. C. Carroll. 2000. Comparison of the TOX A/B test to a cell culture cytotoxicity assay for the detection of Clostridium difficile in stools. Diagn. Microbiol. Infect. Dis. 36:211-213. - PubMed
-
- Barbut, F., D. Decre, V. Lalande, B. Burghoffer, L. Noussair, A. Gigandon, F. Espinasse, L. Raskine, J. Robert, A. Mangeol, C. Branger, and J. C. Petit. 2005. Clinical features of Clostridium difficile-associated diarrhoea due to binary toxin (actin-specific ADP-ribosyltransferase)-producing strains. J. Med. Microbiol. 54:181-185. - PubMed
-
- Bartlett, J. G. 2006. Narrative review: the new epidemic of Clostridium difficile-associated enteric disease. Ann. Intern. Med. 145:758-764. - PubMed
-
- Blake, J. E., F. Mitsikosta, and M. A. Metcalfe. 2004. Immunological detection and cytotoxic properties of toxins from toxin A-positive, toxin B-positive Clostridium difficile variants. J. Med. Microbiol. 53:197-205. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases