Siderophore-based iron acquisition and pathogen control
- PMID: 17804665
- PMCID: PMC2168645
- DOI: 10.1128/MMBR.00012-07
Siderophore-based iron acquisition and pathogen control
Abstract
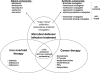
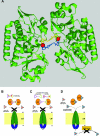
High-affinity iron acquisition is mediated by siderophore-dependent pathways in the majority of pathogenic and nonpathogenic bacteria and fungi. Considerable progress has been made in characterizing and understanding mechanisms of siderophore synthesis, secretion, iron scavenging, and siderophore-delivered iron uptake and its release. The regulation of siderophore pathways reveals multilayer networks at the transcriptional and posttranscriptional levels. Due to the key role of many siderophores during virulence, coevolution led to sophisticated strategies of siderophore neutralization by mammals and (re)utilization by bacterial pathogens. Surprisingly, hosts also developed essential siderophore-based iron delivery and cell conversion pathways, which are of interest for diagnostic and therapeutic studies. In the last decades, natural and synthetic compounds have gained attention as potential therapeutics for iron-dependent treatment of infections and further diseases. Promising results for pathogen inhibition were obtained with various siderophore-antibiotic conjugates acting as "Trojan horse" toxins and siderophore pathway inhibitors. In this article, general aspects of siderophore-mediated iron acquisition, recent findings regarding iron-related pathogen-host interactions, and current strategies for iron-dependent pathogen control will be reviewed. Further concepts including the inhibition of novel siderophore pathway targets are discussed.
Figures




References
-
- Actis, L. A., M. E. Tolmasky, L. M. Crosa, and J. H. Crosa. 1995. Characterization and regulation of the expression of FatB, an iron transport protein encoded by the pJM1 virulence plasmid. Mol. Microbiol. 17:197-204. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

