The missing piece of the type II fatty acid synthase system from Mycobacterium tuberculosis
- PMID: 17804795
- PMCID: PMC1976197
- DOI: 10.1073/pnas.0704132104
The missing piece of the type II fatty acid synthase system from Mycobacterium tuberculosis
Abstract
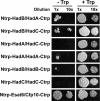
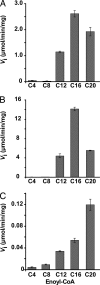
The Mycobacterium tuberculosis fatty acid synthase type II (FAS-II) system has the unique property of producing unusually long-chain fatty acids involved in the biosynthesis of mycolic acids, key molecules of the tubercle bacillus. The enzyme(s) responsible for dehydration of (3R)-hydroxyacyl-ACP during the elongation cycles of the mycobacterial FAS-II remained unknown. This step is classically catalyzed by FabZ- and FabA-type enzymes in bacteria, but no such proteins are present in mycobacteria. Bioinformatic analyses and an essentiality study allowed the identification of a candidate protein cluster, Rv0635-Rv0636-Rv0637. Its expression in recombinant Escherichia coli strains leads to the formation of two heterodimers, Rv0635-Rv0636 (HadAB) and Rv0636-Rv0637 (HadBC), which also occurs in Mycobacterium smegmatis, as shown by split-Trp assays. Both heterodimers exhibit the enzymatic properties expected for mycobacterial FAS-II dehydratases: a marked specificity for both long-chain (>or=C(12)) and ACP-linked substrates. Furthermore, they function as 3-hydroxyacyl dehydratases when coupled with MabA and InhA enzymes from the M. tuberculosis FAS-II system. HadAB and HadBC are the long-sought (3R)-hydroxyacyl-ACP dehydratases. The correlation between the substrate specificities of these enzymes, the organization of the orthologous gene cluster in different Corynebacterineae, and the structure of their mycolic acids suggests distinct roles for both heterodimers during the elongation process. This work describes bacterial monofunctional (3R)-hydroxyacyl-ACP dehydratases belonging to the hydratase 2 family. Their original structure and the fact that they are essential for M. tuberculosis survival make these enzymes very good candidates for the development of antimycobacterial drugs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

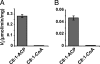


Similar articles
-
Protein-protein interactions within the Fatty Acid Synthase-II system of Mycobacterium tuberculosis are essential for mycobacterial viability.Mol Microbiol. 2004 Dec;54(5):1161-72. doi: 10.1111/j.1365-2958.2004.04334.x. Mol Microbiol. 2004. PMID: 15554959
-
The biosynthesis of mycolic acids in Mycobacterium tuberculosis relies on multiple specialized elongation complexes interconnected by specific protein-protein interactions.J Mol Biol. 2005 Nov 4;353(4):847-58. doi: 10.1016/j.jmb.2005.09.016. Epub 2005 Sep 23. J Mol Biol. 2005. PMID: 16213523
-
Negative regulation by Ser/Thr phosphorylation of HadAB and HadBC dehydratases from Mycobacterium tuberculosis type II fatty acid synthase system.Biochem Biophys Res Commun. 2011 Sep 2;412(3):401-6. doi: 10.1016/j.bbrc.2011.07.051. Epub 2011 Jul 27. Biochem Biophys Res Commun. 2011. PMID: 21819969
-
Product diversity and regulation of type II fatty acid synthases.Biochem Cell Biol. 2004 Feb;82(1):145-55. doi: 10.1139/o03-076. Biochem Cell Biol. 2004. PMID: 15052334 Review.
-
The Molecular Genetics of Mycolic Acid Biosynthesis.Microbiol Spectr. 2014 Aug;2(4):MGM2-0003-2013. doi: 10.1128/microbiolspec.MGM2-0003-2013. Microbiol Spectr. 2014. PMID: 26104214 Review.
Cited by
-
Point mutations within the fatty acid synthase type II dehydratase components HadA or HadC contribute to isoxyl resistance in Mycobacterium tuberculosis.Antimicrob Agents Chemother. 2013 Jan;57(1):629-32. doi: 10.1128/AAC.01972-12. Epub 2012 Oct 31. Antimicrob Agents Chemother. 2013. PMID: 23114755 Free PMC article.
-
The Non-Essential Mycolic Acid Biosynthesis Genes hadA and hadC Contribute to the Physiology and Fitness of Mycobacterium smegmatis.PLoS One. 2015 Dec 23;10(12):e0145883. doi: 10.1371/journal.pone.0145883. eCollection 2015. PLoS One. 2015. PMID: 26701652 Free PMC article.
-
Diversion of phagosome trafficking by pathogenic Rhodococcus equi depends on mycolic acid chain length.Cell Microbiol. 2013 Mar;15(3):458-73. doi: 10.1111/cmi.12050. Epub 2012 Nov 13. Cell Microbiol. 2013. PMID: 23078612 Free PMC article.
-
Active site comparisons and catalytic mechanisms of the hot dog superfamily.Chem Rev. 2013 Mar 13;113(3):2182-204. doi: 10.1021/cr300169a. Epub 2012 Dec 3. Chem Rev. 2013. PMID: 23205964 Free PMC article. Review. No abstract available.
-
Involvement of two latex-clearing proteins during rubber degradation and insights into the subsequent degradation pathway revealed by the genome sequence of Gordonia polyisoprenivorans strain VH2.Appl Environ Microbiol. 2012 Apr;78(8):2874-87. doi: 10.1128/AEM.07969-11. Epub 2012 Feb 10. Appl Environ Microbiol. 2012. PMID: 22327575 Free PMC article.
References
-
- World Health Organization. Anti-Tuberculosis Drug Resistance in the World, Report No. 3. Geneva: WHO; 2004.
-
- Barry CE, III, Lee RE, Mdluli K, Sampson AE, Schroeder BG, Slayden RA, Yuan Y. Prog Lipid Res. 1998;37:143–179. - PubMed
-
- Dubnau E, Chan J, Raynaud C, Mohan VP, Lanéelle MA, Yu K, Quémard A, Smith I, Daffé M. Mol Microbiol. 2000;36:630–637. - PubMed
-
- Bloch K. Adv Enzymol Relat Areas Mol Biol. 1977;45:1–84. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

