Monitoring of lipid storage in Caenorhabditis elegans using coherent anti-Stokes Raman scattering (CARS) microscopy
- PMID: 17804796
- PMCID: PMC1976189
- DOI: 10.1073/pnas.0703594104
Monitoring of lipid storage in Caenorhabditis elegans using coherent anti-Stokes Raman scattering (CARS) microscopy
Abstract
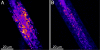
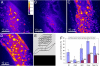
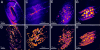
Better understanding of the fundamental mechanisms behind metabolic diseases requires methods to monitor lipid stores on single-cell level in vivo. We have used Caenorhabditis elegans as a model organism to demonstrate the limitations of fluorescence microscopy for imaging of lipids compared with coherent anti-Stokes Raman scattering (CARS) microscopy, the latter allowing chemically specific and label-free imaging in living organisms. CARS microscopy was used to quantitatively monitor the impact of genetic variations in metabolic pathways on lipid storage in 60 specimens of C. elegans. We found that the feeding-defective mutant pha-3 contained a lipid volume fraction one-third of that found in control worms. In contrast, mutants (daf-2, daf-4 dauer) with deficiencies in the insulin and transforming growth factors (IGF and TGF-beta) signaling pathways had lipid volume fractions that were 1.4 and 2 times larger than controls, respectively. This was observed as an accumulation of small-sized lipid droplets in the hypodermal cells, hosting as much as 40% of the total lipid volume in contrast to the 9% for the wild-type larvae. Spectral CARS microscopy measurements indicated that this is accompanied by a shift in the ordering of the lipids from gel to liquid phase. We conclude that the degree of hypodermal lipid storage and the lipid phase can be used as a marker of lipid metabolism shift. This study shows that CARS microscopy has the potential to become a sensitive and important tool for studies of lipid storage mechanisms, improving our understanding of phenomena underlying metabolic disorders.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Spiegelman BM, Flier JS. Cell. 2001;104:531–543. - PubMed
-
- Rosen ED, Spiegelman BM. Annu Rev Cell Dev Biol. 2000;16:145–171. - PubMed
-
- Ashrafi K, Chang FY, Watts JL, Fraser AG, Kamath RS, Ahringer J, Ruvkun G. Nature. 2003;421:268–272. - PubMed
-
- Kimura KD, Tissenbaum HA, Liu Y, Ruvkun G. Science. 1997;277:942–946. - PubMed
-
- Maier O, Oberle V, Hoekstra D. Chem Phys Lipids. 2002;116:3–18. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

