R-Spondin1 regulates Wnt signaling by inhibiting internalization of LRP6
- PMID: 17804805
- PMCID: PMC1965484
- DOI: 10.1073/pnas.0702305104
R-Spondin1 regulates Wnt signaling by inhibiting internalization of LRP6
Abstract
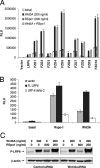
The R-Spondin (RSpo) family of secreted proteins act as potent activators of the Wnt/beta-catenin signaling pathway. We have previously shown that RSpo proteins can induce proliferative effects on the gastrointestinal epithelium in mice. Here we provide a mechanism whereby RSpo1 regulates cellular responsiveness to Wnt ligands by modulating the cell-surface levels of the coreceptor LRP6. We show that RSpo1 activity critically depends on the presence of canonical Wnt ligands and LRP6. Although RSpo1 does not directly activate LRP6, it interferes with DKK1/Kremen-mediated internalization of LRP6 through an interaction with Kremen, resulting in increased LRP6 levels on the cell surface. Our results support a model in which RSpo1 relieves the inhibition DKK1 imposes on the Wnt pathway.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Logan CY, Nusse R. Annu Rev Cell Dev Biol. 2004;20:781–810. - PubMed
-
- Moon RT, Kohn AD, De Ferrari GV, Kaykas A. Nat Rev Genet. 2004;5:691–701. - PubMed
-
- Bejsovec A. Cell. 2005;120:11–14. - PubMed
-
- Gordon MD, Nusse R. J Biol Chem. 2006;281:22429–22433. - PubMed
-
- He X, Semenov M, Tamai K, Zeng X. Development (Cambridge, UK) 2004;131:1663–1677. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

