Translating the IHE Teaching File and Clinical Trial Export (TCE) profile document templates into functional DICOM structured report objects
- PMID: 17805930
- PMCID: PMC3043848
- DOI: 10.1007/s10278-007-9052-5
Translating the IHE Teaching File and Clinical Trial Export (TCE) profile document templates into functional DICOM structured report objects
Abstract
The Integrating the Healthcare Enterprise (IHE) Teaching File and Clinical Trial Export (TCE) integration profile describes a standard workflow for exporting key images from an image manager/archive to a teaching file, clinical trial, or electronic publication application. Two specific digital imaging and communication in medicine (DICOM) structured reports (SR) reference the key images and contain associated case information. This paper presents step-by-step instructions for translating the TCE document templates into functional and complete DICOM SR objects. Others will benefit from these instructions in developing TCE compliant applications.
Figures
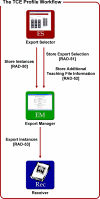








References
-
- Siegel EL, Channin DS. Integrating the healthcare enterprise: a primer. Part 1. Introduction. Radiographics. 2001;21(5):1339–1341. - PubMed
-
- Committee IRT: Teaching file and clinical trial export (TCE). Available at: http://www.ihe.net/Technical_Framework/upload/IHE_TF_Suppl_Teaching_File.... Accessed May 26, 2007, pp 26, 39–40 (Table Q-21)
-
- NEMA: DICOM Part 3: Information Object Definitions (PS 3.3-2004). Available at: ftp://medical.nema.org/medical/dicom/2007/07_03pu.pdf. Accessed May 26, 2007, pp 167, 173 (Section A.135.164), 759, 764, 773, 779–780
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

