Preclinical evaluation of targeting the Nrf2 pathway by triterpenoids (CDDO-Im and CDDO-Me) for protection from LPS-induced inflammatory response and reactive oxygen species in human peripheral blood mononuclear cells and neutrophils
- PMID: 17822364
- PMCID: PMC2396226
- DOI: 10.1089/ars.2007.1745
Preclinical evaluation of targeting the Nrf2 pathway by triterpenoids (CDDO-Im and CDDO-Me) for protection from LPS-induced inflammatory response and reactive oxygen species in human peripheral blood mononuclear cells and neutrophils
Abstract
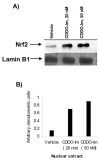
Sepsis is characterized by an inappropriate host immune-inflammatory response and sustained oxidative damage. Nrf2, a bZIP oxidant-responsive transcription factor, regulates a battery of cytoprotective genes including antioxidants and maintains cellular redox homeostasis. Mouse studies have demonstrated a critical role of Nrf2 in improving survival during sepsis. This preclinical ex vivo study using neutrophils and peripheral blood mononuclear cells (PBMCs) as a surrogate cells evaluates the efficacy of CDDO-Im and CDDO-Me [imidazole and methyl ester derivative of 2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oic acid (CDDO)] to activate the Nrf2 pathway and protect from lipopolysaccharide (LPS)-induced inflammatory response in humans. CDDO-Im treatment significantly induced Nrf2-dependent antioxidative genes (HO-1, GCLC, GCLM, and NQO1) in PBMCs isolated from six normal subjects. CDDO-Im increased nuclear accumulation of Nrf2 protein. Pretreatment of PBMC by CDDO-Im significantly attenuated LPS-induced cytokine expression. Similar increases in levels of antioxidant genes and suppression of LPS-induced cytokine expression was observed after CDDO-Me pretreatment. CDDO-Im also greatly inhibited LPS, fMLP, TNF-alpha, and TPA-induced ROS generation in neutrophils. In conclusion, these results demonstrate that activation of the Nrf2-dependent antioxidative pathway by CDDO-Im or CDDO-Me protects against the LPS-induced inflammatory response and suggest that they can be potential therapeutic candidates for intervening sepsis syndrome.
Figures





References
-
- Abraham E. Nuclear factor-kappaB and its role in sepsis-associated organ failure. J Infect Dis. 2003;187(suppl 2):S364–S369. - PubMed
-
- Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. - PubMed
-
- Asehnoune K, Strassheim D, Mitra S, Kim JY, Abraham E. Involvement of reactive oxygen species in toll-like receptor 4-dependent activation of NF-kappaB. J Immunol. 2004;172:2522–2529. - PubMed
-
- Cho HY, Jedlicka AE, Reddy SP, Kensler TW, Yamamoto M, Zhang LY, Kleeberger SR. Role of NRF2 in protection against hyperoxic lung injury in mice. Am J Respir Cell Mol Biol. 2002;26:175–82. - PubMed
-
- Cho HY, Reddy SP, Yamamoto M, Kleeberger SR. The transcription factor NRF2 protects against pulmonary fibrosis. FASEB J. 2004;18:1258–1260. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

