Heme oxygenase-1 in tumors: is it a false friend?
- PMID: 17822372
- PMCID: PMC2096718
- DOI: 10.1089/ars.2007.1659
Heme oxygenase-1 in tumors: is it a false friend?
Abstract
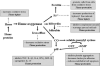
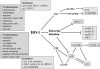
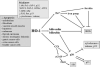
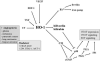
Heme oxygenase-1 (HO-1) catalyzes the oxidation of heme to biologically active products: carbon monoxide (CO), biliverdin, and ferrous iron. It participates in maintaining cellular homeostasis and plays an important protective role in the tissues by reducing oxidative injury, attenuating the inflammatory response, inhibiting cell apoptosis, and regulating cell proliferation. HO-1 is also an important proangiogenic mediator. Most studies have focused on the role of HO-1 in cardiovascular diseases, in which its significant, beneficial activity is well recognized. A growing body of evidence indicates, however, that HO-1 activation may play a role in carcinogenesis and can potently influence the growth and metastasis of tumors. HO-1 is very often upregulated in tumor tissues, and its expression is further increased in response to therapies. Although the exact effect can be tissue specific, HO-1 can be regarded as an enzyme facilitating tumor progression. Accordingly, inhibition of HO-1 can be suggested as a potential therapeutic approach sensitizing tumors to radiation, chemotherapy, or photodynamic therapy.
Figures

References
-
- Aghi M, Cohen KS, Klein RJ, Scadden DT, Chiocca EA. Tumor stromal-derived factor-1 recruits vascular progenitors to mitotic neovasculature, where microenviroment influences their differentiated phenotypes. Cancer Res. 2006;66:9054–9064. - PubMed
-
- Aizawa T, Ishizaka N, Kurokawa K, Nagai R, Nakajima H, Taguchi J, Ohno M. Different effects of angiotensin II and catecholamine on renal cell apoptosis and proliferation in rats. Kidney Int. 2001;59:645–653. - PubMed
-
- Alam J, Shibahara S, Smith A. Transcriptional activation of the heme oxygenase gene by heme and cadmium in mouse hepatoma cells. J Biol Chem. 1989;264:6371–6375. - PubMed
-
- Amin DN, Hida K, Bielenberg DR, Klagsbrun M. Tumor endothelial cells express epidermal growth factor receptor (EGFR) but not ErbB3 and are responsive to EGF and to EGFR kinase inhibitors. Cancer Res. 2006;66:2173–2180. - PubMed
-
- Andreadi CK, Howells LM, Atherfold PA, Manson MM. Involvement of Nrf2, p38, B-Raf, and nuclear factor-kappaB, but not phosphatidylinositol 3-kinase, in induction of hemeoxygenase-1 by dietary polyphenols. Mol Pharmacol. 2006;69:1033–1040. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

