Presynaptic regulation of dendrodendritic dopamine transmission
- PMID: 17822435
- PMCID: PMC3633601
- DOI: 10.1111/j.1460-9568.2007.05775.x
Presynaptic regulation of dendrodendritic dopamine transmission
Abstract
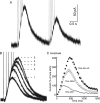
The amount of dopamine release from terminals in the forebrain following an electrical stimulus is variable. This dynamic regulation, both between and within trains of electrical stimuli, has fostered the notion that burst firing of dopamine neurons in vivo may be a determinant of dopamine release in projection areas. In the present study dendritic dopamine release was examined in the substantia nigra and ventral tegmental area in mouse brain slices using whole-cell recording of a dopamine-mediated inhibitory postsynaptic current (IPSC). Paired stimuli produced a depression of the IPSC that was not observed with paired pulses of exogenously applied dopamine. Increasing the number of electrical stimuli from one to five produced an increase in the amplitude the dopamine IPSC but the increase was less than additive, indicating a depression of transmission with each successive stimulus. Analysis with fast-scan cyclic voltammetry demonstrated that presynaptic D2-autoreceptors did not contribute to the depression. Facilitation of the IPSC was observed only after the probability of release was reduced. Thus the regulation of dopamine release in the cell body region was dependent on dopamine neuron impulse activity. Under circumstance where there was initially little activity the probability of dopamine release was high and repetitive activation resulted in depression of further release. With increased activity, the release probability decreased and a burst of activity caused a relative facilitation of dopamine release. This form of regulation would be expected to limit activity within the cell body region.
Figures







References
-
- Beckstead MJ, Grandy DK, Wickman K, Williams JT. Vesicular dopamine release elicits an inhibitory postsynaptic current in midbrain dopamine neurons. Neuron. 2004;42:939–946. - PubMed
-
- Bigornia L, Allen CN, Jan CR, Lyon RA, Titeler M, Schneider AS. D2 dopamine receptors modulate calcium channel currents and catecholamine secretion in bovine adrenal chromaffin cells. J. Pharmacol. Exp. Ther. 1990;252:586–592. - PubMed
-
- Bjorklund A, Lindvall O. Dopamine in dendrites of substantia nigra neurons: suggestions for a role in dendritic terminals. Brain Res. 1975;83:531–537. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

