Recruitment and retention of low-income minority women in a behavioral intervention to reduce smoking, depression, and intimate partner violence during pregnancy
- PMID: 17822526
- PMCID: PMC2020481
- DOI: 10.1186/1471-2458-7-233
Recruitment and retention of low-income minority women in a behavioral intervention to reduce smoking, depression, and intimate partner violence during pregnancy
Abstract
Background: Researchers have frequently encountered difficulties in the recruitment and retention of minorities resulting in their under-representation in clinical trials. This report describes the successful strategies of recruitment and retention of African Americans and Latinos in a randomized clinical trial to reduce smoking, depression and intimate partner violence during pregnancy. Socio-demographic characteristics and risk profiles of retained vs. non-retained women and lost to follow-up vs. dropped-out women are presented. In addition, subgroups of pregnant women who are less (more) likely to be retained are identified.
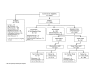
Methods: Pregnant African American women and Latinas who were Washington, DC residents, aged 18 years or more, and of 28 weeks gestational age or less were recruited at six prenatal care clinics. Potentially eligible women were screened for socio-demographic eligibility and the presence of the selected behavioral and psychological risks using an Audio Computer-Assisted Self-Interview. Eligible women who consented to participate completed a baseline telephone evaluation after which they were enrolled in the study and randomly assigned to either the intervention or the usual care group.
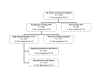
Results: Of the 1,398 eligible women, 1,191 (85%) agreed to participate in the study. Of the 1,191 women agreeing to participate, 1,070 completed the baseline evaluation and were enrolled in the study and randomized, for a recruitment rate of 90%. Of those enrolled, 1,044 were African American women. A total of 849 women completed the study, for a retention rate of 79%. Five percent dropped out and 12% were lost-to-follow up. Women retained in the study and those not retained were not statistically different with regard to socio-demographic characteristics and the targeted risks. Retention strategies included financial and other incentives, regular updates of contact information which was tracked and monitored by a computerized data management system available to all project staff, and attention to cultural competence with implementation of study procedures by appropriately selected, trained, and supervised staff. Single, less educated, alcohol and drug users, non-working, and non-WIC women represent minority women with expected low retention rates.
Conclusion: We conclude that with targeted recruitment and retention strategies, minority women will participate at high rates in behavioral clinical trials. We also found that women who drop out are different from women who are lost to follow-up, and require different strategies to optimize their completion of the study.
Figures
References
-
- Shavers-Hornaday VL, Lynch CF, Burmeister LF, Torner JC. Why are African Americans under-represented in medical research studies? Impediments to participation. Ethn Health. 1997;2:31–45. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical