Deletion of Tgfbr2 in Prx1-cre expressing mesenchyme results in defects in development of the long bones and joints
- PMID: 17822689
- PMCID: PMC2042108
- DOI: 10.1016/j.ydbio.2007.07.040
Deletion of Tgfbr2 in Prx1-cre expressing mesenchyme results in defects in development of the long bones and joints
Abstract
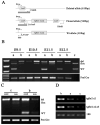
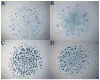
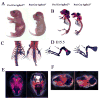
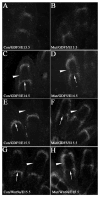
In this study, we address the function of Transforming Growth Factor beta (TGF-beta) and its type II receptor (Tgfbr2) in limb development in vivo. Mouse embryos were generated in which the Tgfbr2 gene was deleted in early limb mesenchyme using Prx1Cre-mediated LoxP recombination. A high level of Tgfbr2 gene deletion was verified in limb mesenchyme by PCR between E9.5 and E10.5 days in Cre expressing mice. RT-PCR assays indicated a significant depletion of Tgfbr2 mRNA by E10.5 days as a result of Cre mediated gene deletion. Furthermore, limb mesenchyme from Cre(+);Tgfbr2(f/f) mice placed in micromass culture did not respond to exogenously added TGF-beta1 confirming the functional deletion of the receptor. However, there was an unexpected increase in the number and intensity of Alcian blue stained chondrogenic nodules in micromass cultures derived from Tgfbr2-deleted limbs relative to cultures from control limbs suggesting that Tgfbr2 normally limits chondrogenesis in vitro. In vivo, early limb development and chondrocyte differentiation occurred normally in Tgfbr2-depleted mice. Later in development, depletion of Tgfbr2 in limb mesenchyme resulted in short limbs and fusion of the joints in the phalanges. Alteration in the length of the long bones was primarily due to a decrease in chondrocyte proliferation after E13.5 days. In addition, the transition from prehypertrophic to hypertrophic cells was accelerated while there was a delay in late hypertrophic differentiation leading to a reduction in the length of the marrow cavity. In the joint, cartilage cells replaced interzone cells during development. Analysis of markers for joint development indicated that the joint was specified properly and that the interzone cells were initially formed but not maintained. The results suggest that Tgfbr2 is required for normal development of the skeleton and that Tgfbr2 can act to limit chondrogenesis in mesenchymal cells like the interzone.
Figures





Similar articles
-
Tgfbr2 is required for development of the skull vault.Dev Biol. 2009 Oct 15;334(2):481-90. doi: 10.1016/j.ydbio.2009.08.015. Epub 2009 Aug 21. Dev Biol. 2009. PMID: 19699732 Free PMC article.
-
Conditional deletion of the TGF-beta type II receptor in Col2a expressing cells results in defects in the axial skeleton without alterations in chondrocyte differentiation or embryonic development of long bones.Dev Biol. 2004 Dec 1;276(1):124-42. doi: 10.1016/j.ydbio.2004.08.027. Dev Biol. 2004. PMID: 15531369
-
Joint TGF-β type II receptor-expressing cells: ontogeny and characterization as joint progenitors.Stem Cells Dev. 2013 May 1;22(9):1342-59. doi: 10.1089/scd.2012.0207. Epub 2013 Feb 15. Stem Cells Dev. 2013. PMID: 23231014 Free PMC article.
-
Conditional deletion of Tgfbr2 in hypertrophic chondrocytes delays terminal chondrocyte differentiation.Matrix Biol. 2012 Jul;31(6):352-9. doi: 10.1016/j.matbio.2012.07.002. Epub 2012 Jul 31. Matrix Biol. 2012. PMID: 22885149
-
Inactivation of Tgfbr2 in Osterix-Cre expressing dental mesenchyme disrupts molar root formation.Dev Biol. 2013 Oct 1;382(1):27-37. doi: 10.1016/j.ydbio.2013.08.003. Epub 2013 Aug 8. Dev Biol. 2013. PMID: 23933490 Free PMC article.
Cited by
-
Impaired Tertiary Dentin Secretion after Shallow Injury in Tgfbr2-Deficient Dental Pulp Cells Is Rescued by Extended CGRP Signaling.Int J Mol Sci. 2024 Jun 21;25(13):6847. doi: 10.3390/ijms25136847. Int J Mol Sci. 2024. PMID: 38999956 Free PMC article.
-
Lung fibroblasts express a miR-19a-19b-20a sub-cluster to suppress TGF-β-associated fibroblast activation in murine pulmonary fibrosis.Sci Rep. 2018 Nov 9;8(1):16642. doi: 10.1038/s41598-018-34839-0. Sci Rep. 2018. PMID: 30413725 Free PMC article.
-
TGF-β Family Signaling in Mesenchymal Differentiation.Cold Spring Harb Perspect Biol. 2018 May 1;10(5):a022202. doi: 10.1101/cshperspect.a022202. Cold Spring Harb Perspect Biol. 2018. PMID: 28507020 Free PMC article. Review.
-
Tgfβ signaling stimulates glycolysis to promote the genesis of synovial joint interzone in developing mouse embryonic limbs.Sci Adv. 2025 Jan 10;11(2):eadq4991. doi: 10.1126/sciadv.adq4991. Epub 2025 Jan 8. Sci Adv. 2025. PMID: 39772668 Free PMC article.
-
The roles and regulatory mechanisms of TGF-β and BMP signaling in bone and cartilage development, homeostasis and disease.Cell Res. 2024 Feb;34(2):101-123. doi: 10.1038/s41422-023-00918-9. Epub 2024 Jan 24. Cell Res. 2024. PMID: 38267638 Free PMC article. Review.
References
-
- Alvarez J, Horton J, Sohn P, Serra R. The perichondrium plays an important role in mediating the effects of TGF-beta1 on endochondral bone formation. Dev Dyn. 2001;221:311–21. - PubMed
-
- Alvarez J, Sohn P, Zeng X, Doetschman T, Robbins DJ, Serra R. TGFbeta2 mediates the effects of hedgehog on hypertrophic differentiation and PTHrP expression. Development. 2002;129:1913–24. - PubMed
-
- Archer CW, Dowthwaite GP, Francis-West P. Development of synovial joints. Birth Defects Res C Embryo Today. 2003;69:144–55. - PubMed
-
- Baffi MO, Slattery E, Sohn P, Moses HL, Chytil A, Serra R. Conditional deletion of the TGF-beta type II receptor in Col2a expressing cells results in defects in the axial skeleton without alterations in chondrocyte differentiation or embryonic development of long bones. Dev Biol. 2004;276:124–42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

