A gynecologic oncology group randomized phase III trial of whole abdominal irradiation (WAI) vs. cisplatin-ifosfamide and mesna (CIM) as post-surgical therapy in stage I-IV carcinosarcoma (CS) of the uterus
- PMID: 17822748
- PMCID: PMC2752331
- DOI: 10.1016/j.ygyno.2007.07.070
A gynecologic oncology group randomized phase III trial of whole abdominal irradiation (WAI) vs. cisplatin-ifosfamide and mesna (CIM) as post-surgical therapy in stage I-IV carcinosarcoma (CS) of the uterus
Abstract
Purpose: After initial surgery, there has been no established consensus regarding adjunctive therapy for patients with uterine carcinosarcoma (CS). This study was designed to compare patient outcome following treatment with adjuvant whole abdominal irradiation (WAI) versus (vs.) chemotherapy for patients with this rare group of female pelvic malignancies.
Patients and methods: Eligible, consenting women with stage I-IV uterine CS, no more than 1 cm postsurgical residuum and/or no extra-abdominal spread had their treatments randomly assigned as either WAI or three cycles of cisplatin (C), ifosfamide (I), and mesna (M).
Results: 232 patients were enrolled, of whom 206 (WAI=105; CIM=101) were deemed eligible. Patient demographics and characteristics were similar between arms. FIGO stage (both arms) was: I=64 (31%); II=26 (13%); III=92 (45%); IV=24 (12%). The estimated crude probability of recurring within 5 years was 58% (WAI) and 52% (CIM). Adjusting for stage and age, the recurrence rate was 21% lower for CIM patients than for WAI patients (relative hazard [RH]=0.789, 95% confidence interval [CI]: (0.530-1.176), p=0.245, 2-tail test). The estimated death rate was 29% lower among the CIM group (RH=0.712, 95% CI: 0.484-1.048, p=0.085, two-tail test).
Conclusion: We did not find a statistically significant advantage in recurrence rate or survival for adjuvant CIM over WAI in patients with uterine CS. However, the observed differences favor the use of combination chemotherapy in future trials.
Figures

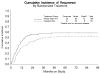
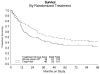


Comment in
-
Eleven years: the long and winding road.Gynecol Oncol. 2007 Nov;107(2):166-8. doi: 10.1016/j.ygyno.2007.09.032. Gynecol Oncol. 2007. PMID: 17950382 No abstract available.
References
-
- [November 10, 2006]. http://www.cancer.org/docroot/home/index.asp.
-
- Curtin JP, Kavanagh JJ, Fox H, et al. Corpus: Mesenchymal tumors. In: Hoskins WJ, Perez CA, Young RC, editors. Principles and Practice of Gynecologic Oncology. 3. Lippincott, Williams & Wilkins; Philadelphia: 2000. p. 961.
-
- Menczer J, Levy T, Piura B, et al. A comparison between different postoperative treatment modalities of uterine carcinosarcoma. Gynecol Oncol. 2005;97:166–70. - PubMed
-
- McCluggage WG. Uterine carcinosarcomas (malignant mixed Mullerian tumors) are metaplastic carcinosarcomas. Int J Gynecol Cancer. 2002;12:687–90. - PubMed
-
- WHO. Pathology and genetics of tumors of the breast and female genital organs. Lyon: IARC Press; 2003.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

