The unfolded protein response of B-lymphocytes: PERK-independent development of antibody-secreting cells
- PMID: 17822768
- PMCID: PMC2677759
- DOI: 10.1016/j.molimm.2007.07.029
The unfolded protein response of B-lymphocytes: PERK-independent development of antibody-secreting cells
Abstract
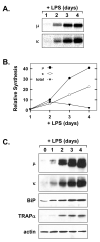
When B-lymphocytes differentiate into plasma cells, immunoglobulin (Ig) heavy and light chain synthesis escalates and the entire secretory apparatus expands to support high-rate antibody secretion. These same events occur when murine B-cells are stimulated with lipopolysaccharide (LPS), providing an in vitro model in which to investigate the differentiation process. The unfolded protein response (UPR), a multi-pathway signaling response emanating from the endoplasmic reticulum (ER) membrane, allows cells to adapt to increasing demands on the protein folding capacity of the ER. As such, the UPR plays a pivotal role in the differentiation of antibody-secreting cells. Three specific stress sensors, IRE1, PERK/PEK and ATF6, are central to the recognition of ER stress and induction of the UPR. IRE1 triggers splicing of Xbp-1 mRNA, yielding a transcriptional activator of the UPR termed XBP-1(S), and activation of the IRE1/XBP-1 pathway has been reported to be required for expansion of the ER and antibody secretion. Here, we provide evidence that PERK is not activated in LPS-stimulated splenic B-cells, whereas XBP-1(S) and the UPR transcriptional activator ATF6 are both induced. We further demonstrate that Perk-/- B-cells develop and are fully competent for induction of Ig synthesis and antibody secretion when stimulated with LPS. These data provide clear evidence for differential activation and utilization of distinct UPR components as activated B-lymphocytes increase Ig synthesis and differentiate into specialized secretory cells.
Figures




References
-
- Andersson J, Sjoberg O, Moller G. Induction of immunoglobulin and antibody synthesis in vitro by lipopolysaccharides. Eur. J. Immunol. 1972;2:349–353. - PubMed
-
- Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat. Cell Biol. 2000;2:326–332. - PubMed
-
- Brewer JW, Hendershot LM. Building an antibody factory: a job for the unfolded protein response. Nat. Immunol. 2005;6:23–29. - PubMed
-
- Calame KL, Lin K-I, Tunyaplin C. Regulatory mechanisms that determine the development and function of plasma cells. Annu. Rev. Immunol. 2003;21:205–230. - PubMed
-
- Calfon M, Zeng H, Urano F, Till JH, Hubbard SR, Harding HP, Clark SG, Ron D. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature. 2002;415:92–96. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

