Erythropoietin-receptor agonists in critically ill patients: a meta-analysis of randomized controlled trials
- PMID: 17823140
- PMCID: PMC1976638
- DOI: 10.1503/cmaj.071055
Erythropoietin-receptor agonists in critically ill patients: a meta-analysis of randomized controlled trials
Abstract
Introduction: Anemia and the need for red blood cell transfusions are common among patients admitted to intensive care units. Erythropoietin has been used to decrease the need for transfusions; however, its ability to improve clinical outcomes is unknown. We evaluated the effect of erythropoietin-receptor agonists on clinically important outcomes, including mortality, length of stay in hospital or intensive care unit, ventilator use, transfusion requirements and major adverse events.
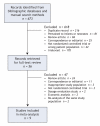
Methods: To identify relevant studies, we searched electronic databases covering 1950 to 2007 (MEDLINE, EMBASE, the Cochrane Central Register of Controlled Trials and the Scopus database). We also searched conference proceedings and grey literature sources. We selected all randomized controlled trials involving critically ill patients that compared an erythropoietin-receptor agonist with a placebo or no intervention. No language restrictions were considered. Data were extracted using a standardized extraction template. We used a fixed effects model to calculate all summary measures of treatment effects.
Results: Of 673 identified records, 9 studies that investigated erythropoietin alpha met the eligibility criteria and were included in our analysis. Erythropoietin, compared with placebo or no intervention, had no statistically significant effect on overall mortality (odds ratio [OR] 0.86, 95% confidence interval [CI] 0.71-1.05, I2 = 0%). The treatment and control groups did not differ in the length of stay in hospital or intensive care unit, or in the duration of mechanical ventilation, in the 3 studies that reported these outcomes. Erythropoietin, compared with placebo, significantly reduced the odds of a patient receiving at least 1 transfusion (OR 0.73, 95% CI 0.64-0.84, I2 = 54.7%). The mean number of units of blood transfused per patient was decreased by 0.41 units in the erythropoietin group (95% CI 0.10-0.74, I2 = 79.2%). Most of the included studies were performed before the widespread adoption of a restrictive transfusion strategy. Only 1 study provided detailed reports of adverse events, and none of the studies systematically evaluated all patients for venous thromboembolism.
Interpretation: At this time, we do not recommend the routine use of erythropoietin-receptor agonists in critically ill patients. The reduction in red blood cell transfusions per patient was very small, and there is insufficient evidence to determine whether this intervention results in clinically important benefits with acceptable risks.
Figures



Comment in
-
Erythropoietin use in critically ill patients: forest and trees.CMAJ. 2007 Sep 25;177(7):747-9. doi: 10.1503/cmaj.071150. Epub 2007 Sep 5. CMAJ. 2007. PMID: 17823138 Free PMC article. No abstract available.
-
Corticosteroids and erythropoeitin-receptor agonists.CMAJ. 2008 Jan 1;178(1):66. doi: 10.1503/cmaj.1070144. CMAJ. 2008. PMID: 18166738 Free PMC article. No abstract available.
Similar articles
-
Efficacy of recombinant human erythropoietin in critically ill patients: a randomized controlled trial.JAMA. 2002 Dec 11;288(22):2827-35. doi: 10.1001/jama.288.22.2827. JAMA. 2002. PMID: 12472324 Clinical Trial.
-
Efficacy and safety of epoetin alfa in critically ill patients.N Engl J Med. 2007 Sep 6;357(10):965-76. doi: 10.1056/NEJMoa071533. N Engl J Med. 2007. PMID: 17804841 Clinical Trial.
-
Erythropoietic agents for anemia of critical illness.Am J Health Syst Pharm. 2008 Mar 15;65(6):540-6. doi: 10.2146/ajhp070225. Am J Health Syst Pharm. 2008. PMID: 18319499 Review.
-
Efficacy of recombinant human erythropoietin in critically ill patients admitted to a long-term acute care facility: a randomized, double-blind, placebo-controlled trial.Crit Care Med. 2006 Sep;34(9):2310-6. doi: 10.1097/01.CCM.0000233873.17954.42. Crit Care Med. 2006. PMID: 16878035 Clinical Trial.
-
Is there a place for epoetin alfa in managing anemia during critical illness?Clin Ther. 2004 Jun;26(6):819-29. doi: 10.1016/s0149-2918(04)90126-9. Clin Ther. 2004. PMID: 15262453 Review.
Cited by
-
Protective effect of erythropoietin against myocardial injury in rats with sepsis and its underlying mechanisms.Mol Med Rep. 2015 May;11(5):3317-29. doi: 10.3892/mmr.2015.3155. Epub 2015 Jan 8. Mol Med Rep. 2015. PMID: 25572660 Free PMC article.
-
Bench to bedside: A role for erythropoietin in sepsis.Crit Care. 2010;14(4):227. doi: 10.1186/cc9049. Epub 2010 Aug 6. Crit Care. 2010. PMID: 20727227 Free PMC article. Review.
-
Anaemia and transfusion triggers in critically ill patients - What we have learnt thus far.J Intensive Care Soc. 2019 Nov;20(4):284-289. doi: 10.1177/1751143718783615. Epub 2018 Jun 25. J Intensive Care Soc. 2019. PMID: 31695732 Free PMC article.
-
Effect of erythropoietin on SOFA score, Glasgow Coma Scale and mortality in traumatic brain injury patients: a randomized-double-blind controlled trial.Ann Med Surg (Lond). 2024 May 15;86(7):3990-3997. doi: 10.1097/MS9.0000000000002143. eCollection 2024 Jul. Ann Med Surg (Lond). 2024. PMID: 38989196 Free PMC article.
-
Indication creep: physician beware.CMAJ. 2007 Sep 25;177(7):697, 699. doi: 10.1503/cmaj.071223. Epub 2007 Sep 5. CMAJ. 2007. PMID: 17823137 Free PMC article. No abstract available.
References
-
- Corwin HL, Gettinger A, Rodriguez RM, et al. Efficacy of recombinant human erythropoietin in the critically ill patient: a randomized, double-blind, placebo-controlled trial. Crit Care Med 1999;27:2346-50. - PubMed
-
- Corwin HL, Gettinger A, Pearl RG, et al. The CRIT Study: anemia and blood transfusion in the critically ill — current clinical practice in the United States. Crit Care Med 2004;32:39-52. - PubMed
-
- Rodriguez RM, Corwin HL, Gettinger A, et al. Nutritional deficiencies and blunted erythropoietin response as causes of the anemia of critical illness. J Crit Care 2001;16:36-41. - PubMed
-
- Vincent JL, Baron JF, Reinhart K, et al. Anemia and blood transfusion in critically ill patients. JAMA 2002;288:1499-507. - PubMed
-
- Smoller BR, Kruskall MS. Phlebotomy for diagnostic laboratory tests in adults. Pattern of use and effect on transfusion requirements. N Engl J Med 1986;314:1233-5. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
