Gating of nicotinic ACh receptors; new insights into structural transitions triggered by agonist binding that induce channel opening
- PMID: 17823204
- PMCID: PMC2276999
- DOI: 10.1113/jphysiol.2007.142554
Gating of nicotinic ACh receptors; new insights into structural transitions triggered by agonist binding that induce channel opening
Abstract
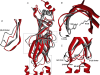
Nicotinic acetylcholine receptors (nAChRs) are in the superfamily of Cys-loop ligand-gated ion channels, and are pentameric assemblies of five subunits, with each subunit arranged around the central ion-conducting pore. The binding of ACh to the extracellular interface between two subunits induces channel opening. With the recent 4 A resolution of the Torpedo nAChR, and the crystal structure of the related molluscan ACh binding protein, much has been learned about the structure of the ligand binding domain and the channel pore, as well as major structural rearrangements that may confer channel opening. For example, the putative pathway coupling agonist binding to channel gating may include a major rearrangement of the C-loop within the ligand binding pocket, and the disruption of a salt bridge between an arginine residue at the end of the beta10 strand and a glutamate residue in the beta1-beta2 linker. Here we will review and discuss the latest structural findings aiming to further refine the transduction pathway linking binding to gating for the nAChR channels, and discuss similarities and differences among the different members of this Cys-loop superfamily of receptors.
Figures
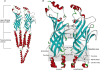
References
-
- Absalom NL, Lewis TM, Kaplan W, Pierce KD, Schofield PR. Role of charged residues in coupling ligand binding and channel activation in the extracellular domain of the glycine receptor. J Biol Chem. 2003;278:50151–50157. - PubMed
-
- Bohler S, Gay S, Bertrand S, Corringer PJ, Edelstein SJ, Changeux JP, Bertrand D. Desensitization of neuronal nicotinic acetylcholine receptors conferred by N-terminal segments of the β2 subunit. Biochemistry. 2001;40:2066–2074. - PubMed
-
- Bouzat C, Gumilar F, Spitzmaul G, Wang HL, Rayes D, Hansen SB, Taylor P, Sine SM. Coupling of agonist binding to channel gating in an ACh-binding protein linked to an ion channel. Nature. 2004;430:896–900. - PubMed
-
- Brejc K, Van Dijk WJ, Klaassen RV, Schuurmans M, Van Der Oost J, Smit AB, Sixma TK. Crystal structure of an ACh-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature. 2001;411:269–276. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

