Changes in synaptic transmission properties due to the expression of N-type calcium channels at the calyx of Held synapse of mice lacking P/Q-type calcium channels
- PMID: 17823210
- PMCID: PMC2277003
- DOI: 10.1113/jphysiol.2007.139683
Changes in synaptic transmission properties due to the expression of N-type calcium channels at the calyx of Held synapse of mice lacking P/Q-type calcium channels
Abstract
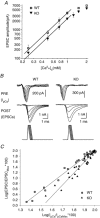
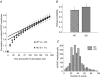
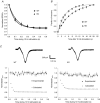
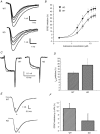
P/Q-type and N-type calcium channels mediate transmitter release at rapidly transmitting central synapses, but the reasons for the specific expression of one or the other in each particular synapse are not known. Using whole-cell patch clamping from in vitro slices of the auditory brainstem we have examined presynaptic calcium currents (I(pCa)) and glutamatergic excitatory postsynaptic currents (EPSCs) at the calyx of Held synapse from transgenic mice in which the alpha(1A) pore-forming subunit of the P/Q-type Ca(2+) channels is ablated (KO). The power relationship between Ca(2+) influx and quantal output was studied by varying the number of Ca(2+) channels engaged in triggering release. Our results have shown that more overlapping Ca(2+) channel domains are required to trigger exocytosis when N-type replace P/Q-type calcium channels suggesting that P/Q type Ca(2+) channels are more tightly coupled to synaptic vesicles than N-type channels, a hypothesis that is verified by the decrease in EPSC amplitudes in KO synapses when the slow Ca(2+) buffer EGTA-AM was introduced into presynaptic calyces. Significant alterations in short-term synaptic plasticity were observed. Repetitive stimulation at high frequency generates short-term depression (STD) of EPSCs, which is not caused by presynaptic Ca(2+) current inactivation neither in WT or KO synapses. Recovery after STD is much slower in the KO than in the WT mice. Synapses from KO mice exhibit reduced or no EPSC paired-pulse facilitation and absence of facilitation in their presynaptic N-type Ca(2+) currents. Simultaneous pre- and postsynaptic double patch recordings indicate that presynaptic Ca(2+) current facilitation is the main determinant of facilitation of transmitter release. Finally, KO synapses reveal a stronger modulation of transmitter release by presynaptic GTP-binding protein-coupled receptors (gamma-aminobutyric acid type B receptors, GABA(B), and adenosine). In contrast, metabotropic glutamate receptors (mGluRs) are not functional at the synapses of these mice. These experiments reinforce the idea that presynaptic Ca(2+) channels expression may be tuned for speed and modulatory control through differential subtype expression.
Figures




Similar articles
-
Presynaptic CaV2.1 calcium channels carrying familial hemiplegic migraine mutation R192Q allow faster recovery from synaptic depression in mouse calyx of Held.J Neurophysiol. 2012 Dec;108(11):2967-76. doi: 10.1152/jn.01183.2011. Epub 2012 Sep 5. J Neurophysiol. 2012. PMID: 22956801 Free PMC article.
-
P/Q-type calcium channel ablation in a mice glycinergic synapse mediated by multiple types of Ca²+ channels alters transmitter release and short term plasticity.Neuroscience. 2011 Sep 29;192:219-30. doi: 10.1016/j.neuroscience.2011.06.021. Epub 2011 Jun 14. Neuroscience. 2011. PMID: 21718757
-
Functional compensation of P/Q by N-type channels blocks short-term plasticity at the calyx of Held presynaptic terminal.J Neurosci. 2004 Nov 17;24(46):10379-83. doi: 10.1523/JNEUROSCI.2104-04.2004. J Neurosci. 2004. PMID: 15548652 Free PMC article.
-
Presynaptic Black Box Opened by Pioneers at Biophysics Department in University College London.Neuroscience. 2020 Jul 15;439:10-21. doi: 10.1016/j.neuroscience.2019.04.029. Epub 2019 May 18. Neuroscience. 2020. PMID: 31108128 Review.
-
Role of Ca(2+) channels in short-term synaptic plasticity.Curr Opin Neurobiol. 2007 Jun;17(3):352-9. doi: 10.1016/j.conb.2007.04.005. Epub 2007 Apr 26. Curr Opin Neurobiol. 2007. PMID: 17466513 Review.
Cited by
-
Calcium channels and short-term synaptic plasticity.J Biol Chem. 2013 Apr 12;288(15):10742-9. doi: 10.1074/jbc.R112.411645. Epub 2013 Feb 11. J Biol Chem. 2013. PMID: 23400776 Free PMC article. Review.
-
Presynaptic CaV2.1 calcium channels carrying familial hemiplegic migraine mutation R192Q allow faster recovery from synaptic depression in mouse calyx of Held.J Neurophysiol. 2012 Dec;108(11):2967-76. doi: 10.1152/jn.01183.2011. Epub 2012 Sep 5. J Neurophysiol. 2012. PMID: 22956801 Free PMC article.
-
Targeting Alternative Splicing as a Potential Therapy for Episodic Ataxia Type 2.Biomedicines. 2020 Sep 5;8(9):332. doi: 10.3390/biomedicines8090332. Biomedicines. 2020. PMID: 32899500 Free PMC article. Review.
-
Interaction between facilitation and depression at a large CNS synapse reveals mechanisms of short-term plasticity.J Neurosci. 2010 Feb 10;30(6):2007-16. doi: 10.1523/JNEUROSCI.4378-09.2010. J Neurosci. 2010. PMID: 20147529 Free PMC article.
-
Synaptic gain-of-function effects of mutant Cav2.1 channels in a mouse model of familial hemiplegic migraine are due to increased basal [Ca2+]i.J Neurosci. 2014 May 21;34(21):7047-58. doi: 10.1523/JNEUROSCI.2526-13.2014. J Neurosci. 2014. PMID: 24849341 Free PMC article.
References
-
- Currie KP, Fox AP. ATP serves as a negative feedback inhibitor of voltage-gated Ca2+ channel currents in cultured bovine adrenal chromaffin cells. Neuron. 1996;16:1027–1036. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

