Factors defining a pacemaker region for synchrony in the hippocampus
- PMID: 17823211
- PMCID: PMC2276992
- DOI: 10.1113/jphysiol.2007.138131
Factors defining a pacemaker region for synchrony in the hippocampus
Abstract
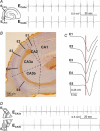
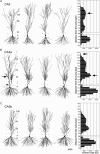
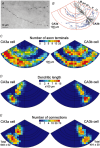
Synchronous activities of neuronal populations are often initiated in a pacemaker region and spread to recruit other regions. Here we examine factors that define a pacemaker site. The CA3a region acts as the pacemaker for disinhibition induced synchrony in guinea pig hippocampal slices and CA3b is a follower region. We found CA3a pyramidal cells were more excitable and fired in bursts more frequently than CA3b cells. CA3a cells had more complex dendritic arbors than CA3b cells especially in zones targetted by recurrent synapses. The product of the density of pyramidal cell axon terminals and dendritic lengths in innervated zones predicted a higher recurrent synaptic connectivity in the CA3a than in the CA3b region. We show that some CA3a cells but few CA3b cells behave as pacemaker cells by firing early during population events and by recruiting follower cells to fire. With a greater excitability and enhanced synaptic connectivity these CA3a cells may also possess initiating functions for other hippocampal ensemble activities initiated in this region.
Figures
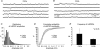




References
-
- Amaral DG, Ishizuka N, Claiborne B. Neurons, numbers and the hippocampal network. Prog Brain Res. 1990;83:1–11. - PubMed
-
- Baimbridge KG, Miller JJ. Immunohistochemical localization of calcium-binding protein in the cerebellum, hippocampal formation and olfactory bulb of the rat. Brain Res. 1982;245:223–229. - PubMed
-
- Bartesaghi R, Severi S. Effects of early environment on field CA3a pyramidal neuron morphology in the guinea-pig. Neuroscience. 2002;110:475–488. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

