A proposed signaling motif for nuclear import in mRNA processing via the formation of arginine claw
- PMID: 17823247
- PMCID: PMC1968059
- DOI: 10.1073/pnas.0703151104
A proposed signaling motif for nuclear import in mRNA processing via the formation of arginine claw
Abstract
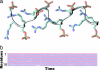
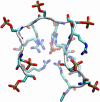
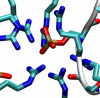
Phosphorylation of proteins by kinases is the most commonly studied class of posttranslational modification, yet its structural consequences are not well understood. The human SR (serine-arginine) protein ASF/SF2 relies on the processive phosphorylation of the serine residues of eight consecutive arginine-serine (RS) dipeptide repeats at the C terminus by SRPK1 before it can be transported into the nucleus. This SR protein plays critical roles in spliceosome assembly, pre-mRNA splicing, and mRNA export, and the phosphorylation process of the RS repeats has been extensively studied experimentally. However, knowledge of the conformational changes associated with the phosphorylation of this simple sequence and how it triggers the importation of the SR protein is lacking. Here, we have carried out extensive molecular dynamics simulations to show that phosphorylation of the eight RS repeats significantly alters the peptide's conformation and leads to the formation of very stable structures that are likely to be involved in the recognition, binding, and transport of the SR protein. Specifically, we found an unusual symmetry-broken phase of conformations of the repetitive and quasi-symmetric phosphorylated peptide sequence. One of the main characteristics of these conformations is the exposed phosphate groups on the periphery, which possibly could serve as the recognition platform for the transport protein transportin-SR2.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Johnson LN, Lewis RJ. Chem Rev. 2001;101:2209–2242. - PubMed
-
- Holmberg CI, Tran SE, Eriksson JE, Sistonen L. Trends Biochem Sci. 2002;27:619–627. - PubMed
-
- Yaffe MB, Schutkowski M, Shen M, Zhou XZ, Stukenberg PT, Rahfeld JU, Xu J, Kuang J, Kirschner MW, Fischer G, et al. Science. 1997;278:1957–1960. - PubMed
-
- Hamelberg D, Shen T, McCammon JA. J Am Chem Soc. 2005;127:1969–1974. - PubMed
-
- Schutkowski M, Bernhardt A, Zhou XZ, Shen M, Reimer U, Rahfeld JU, Lu KP, Fischer G. Biochemistry. 1998;37:5566–5575. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

