Oxidative stress, gene expression, and protein changes induced in the human placenta during labor
- PMID: 17823277
- PMCID: PMC1988867
- DOI: 10.2353/ajpath.2007.070528
Oxidative stress, gene expression, and protein changes induced in the human placenta during labor
Abstract
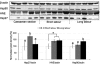
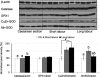
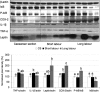
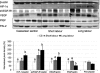
Malperfusion of the placenta has been implicated as a cause of oxidative stress in complications of human pregnancy, leading to release of proinflammatory cytokines and anti-angiogenic factors into the maternal circulation. Uterine contractions during labor are known to be associated with intermittent utero-placental perfusion. We therefore tested whether oxidative stress, proinflammatory cytokines, and angiogenic regulators were increased in placentas subjected to short (<5 hours) and long (>15 hours) labor compared with nonlabored controls delivered by cesarean section. In addition, broader changes in gene transcripts were assessed by microarray analysis. Oxidative stress, activation of the nuclear factor-kappaB pathway, tumor necrosis factor-alpha and interleukin 1beta all increased in placental tissues after labor. Stabilization of hypoxia-inducible factor-1alpha and increased vascular endothelial growth factor soluble receptor-1 were also observed. By contrast, tissue levels of placenta growth factor decreased. Apoptosis was also activated in labored placentas. The magnitude of these changes related to the duration of labor. After labor, 55 gene transcripts were up-regulated and 35 down-regulated, and many of these changes were reflected at the protein level. In conclusion, labor is a powerful inducer of placental oxidative stress, inflammatory cytokines, and angiogenic regulators. Our findings are consistent with intermittent perfusion being the initiating cause. Placentas subjected to labor do not reflect the normal in vivo state at the molecular level.
Figures
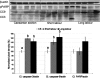

References
-
- Hubel CA. Oxidative stress in the pathogenesis of preeclampsia. Proc Soc Exp Biol Med. 1999;222:222–235. - PubMed
-
- Myatt L, Cui X. Oxidative stress in the placenta. Histochem Cell Biol. 2004;122:369–382. - PubMed
-
- Burton GJ, Jauniaux E. Placental oxidative stress: from miscarriage to preeclampsia. J Soc Gynecol Invest. 2004;11:342–352. - PubMed
-
- Brosens IA. Morphological changes in the utero-placental bed in pregnancy hypertension. Clin Obstet Gynaecol. 1977;4:573–593. - PubMed
-
- Kim YM, Bujold E, Chaiworapongsa T, Gomez R, Yoon BH, Thaler HT, Rotmensch S, Romero R. Failure of physiologic transformation of the spiral arteries in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2003;189:1063–1069. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

