Targeting inflammatory demyelinating lesions to sites of Wallerian degeneration
- PMID: 17823280
- PMCID: PMC2043517
- DOI: 10.2353/ajpath.2007.070147
Targeting inflammatory demyelinating lesions to sites of Wallerian degeneration
Abstract
In Theiler's murine encephalomyelitis virus (TMEV) infection, an animal model for multiple sclerosis (MS), axonal injury precedes inflammatory demyelinating lesions, and the distribution of axonal damage present during the early phase of infection corresponds to regions where subsequent demyelination occurs during the chronic phase. We hypothesized that axonal damage recruits inflammatory cells to sites of Wallerian degeneration, leading to demyelination. Three weeks after TMEV infection, axonal degeneration was induced in the posterior funiculus of mice by injecting the toxic lectin Ricinus communis agglutinin (RCA) I into the sciatic nerve. Neuropathology was examined 1 week after lectin injection. Control mice, infected with TMEV but receiving no RCA I, had inflammatory demyelinating lesions in the anterior/lateral funiculi. Other control mice that received RCA I alone did not develop inflammatory lesions. In contrast, RCA I injection into TMEV-infected mice induced lesions in the posterior funiculus in addition to the anterior/lateral funiculi. We found no differences in lymphoproliferative responses or antibody titers against TMEV among the groups. This suggests that axonal degeneration contributes to the recruitment of inflammatory cells into the central nervous system by altering the local microenvironment. In this scenario, lesions develop from the axon (inside) to the myelin (outside) (Inside-Out model).
Figures


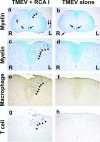
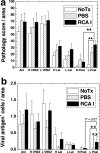
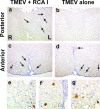

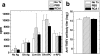
References
-
- Legido A, Tenembaum SN, Katsetos CD, Menkes JH. Autoimmune and postinfectious diseases. Menkes JH, Sarnat HB, Maria BL, editors. Philadelphia: Lippincott Williams & Wilkins,; Child Neurology. (ed 7) 2006:pp 557–657.
-
- Silber E, Sharief MK. Axonal degeneration in the pathogenesis of multiple sclerosis. J Neurol Sci. 1999;170:11–18. - PubMed
-
- Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mörk S, Bö L. Axonal transection in the lesions of multiple sclerosis. N Engl J Med. 1998;338:278–285. - PubMed
-
- Ferguson B, Matyszak MK, Esiri MM, Perry VH. Axonal damage in acute multiple sclerosis lesions. Brain. 1997;120:393–399. - PubMed
-
- Kuhlmann T, Lingfeld G, Bitsch A, Schuchardt J, Brück W. Acute axonal damage in multiple sclerosis is most extensive in early disease stages and decreases over time. Brain. 2002;125:2202–2212. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

