The emerging role of valve interstitial cell phenotypes in regulating heart valve pathobiology
- PMID: 17823281
- PMCID: PMC2043503
- DOI: 10.2353/ajpath.2007.070251
The emerging role of valve interstitial cell phenotypes in regulating heart valve pathobiology
Abstract
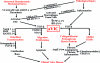
The study of the cellular and molecular pathogenesis of heart valve disease is an emerging area of research made possible by the availability of cultures of valve interstitial cells (VICs) and valve endothelial cells (VECs) and by the design and use of in vitro and in vivo experimental systems that model elements of valve biological and pathobiological activity. VICs are the most common cells in the valve and are distinct from other mesenchymal cell types in other organs. We present a conceptual approach to the investigation of VICs by focusing on VIC phenotype-function relationships. Our review suggests that there are five identifiable phenotypes of VICs that define the current understanding of their cellular and molecular functions. These include embryonic progenitor endothelial/mesenchymal cells, quiescent VICs (qVICs), activated VICs (aVICs), progenitor VICs (pVICs), and osteoblastic VICs (obVICs). Although these may exhibit plasticity and may convert from one form to another, compartmentalizing VIC function into distinct phenotypes is useful in bringing clarity to our understanding of VIC pathobiology. We present a conceptual model that is useful in the design and interpretation of studies on the function of an important phenotype in disease, the activated VIC. We hope this review will inspire members of the investigative pathology community to consider valve pathobiology as an exciting new frontier exploring pathogenesis and discovering new therapeutic targets in cardiovascular diseases.
Figures



References
-
- Lester W, Rosenthal A, Granton B, Gotlieb AI. Porcine mitral valve interstitial cells in culture. Lab Invest. 1988;59:710–719. - PubMed
-
- Lester WM, Gotlieb AI. In vitro repair of the wounded porcine mitral valve. Circ Res. 1988;62:833–845. - PubMed
-
- Mulholland DL, Gotlieb AI. Cell biology of valvular interstitial cells. Can J Cardiol. 1996;12:231–236. - PubMed
-
- Butany J, Gotlieb AI. Native valvular heart disease. McManus BM, editor. Philadelphia: Current Medicine Inc.; Atlas of Cardiovascular Pathology for the Clinician. (ed 2) 2001:pp 201–215.
-
- Durbin A, Gotlieb AI. Advances towards understanding heart valve response to injury. Cardiovasc Pathol. 2002;11:69–77. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

