Dissecting the impact of chemotherapy on the human hair follicle: a pragmatic in vitro assay for studying the pathogenesis and potential management of hair follicle dystrophy
- PMID: 17823286
- PMCID: PMC1988866
- DOI: 10.2353/ajpath.2007.061164
Dissecting the impact of chemotherapy on the human hair follicle: a pragmatic in vitro assay for studying the pathogenesis and potential management of hair follicle dystrophy
Abstract
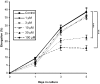
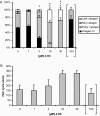
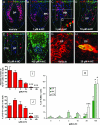
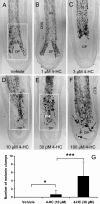
Chemotherapy-induced alopecia represents one of the major unresolved problems of clinical oncology. The underlying molecular pathogenesis in humans is virtually unknown because of the lack of adequate research models. Therefore, we have explored whether microdissected, organ-cultured, human scalp hair follicles (HFs) in anagen VI can be exploited for dissecting and manipulating the impact of chemotherapy on human HFs. Here, we show that these organ-cultured HFs respond to a key cyclophosphamide metabolite, 4-hydroperoxycyclophosphamide (4-HC), in a manner that resembles chemotherapy-induced HF dystrophy as it occurs in vivo: namely, 4-HC induced melanin clumping and melanin incontinence, down-regulated keratinocyte proliferation, massively up-regulated apoptosis of hair matrix keratinocytes, prematurely induced catagen, and up-regulated p53. In addition, 4-HC induced DNA oxidation and the mitochondrial DNA common deletion. The organ culture system facilitated the identification of new molecular targets for chemotherapy-induced HF damage by microarray technology (eg, interleukin-8, fibroblast growth factor-18, and glypican 6). It was also used to explore candidate chemotherapy protectants, for which we used the cytoprotective cytokine keratinocyte growth factor as exemplary pilot agent. Thus, this novel system serves as a powerful yet pragmatic tool for dissecting and manipulating the impact of chemotherapy on the human HF.
Figures


References
-
- Hesketh PJ, Batchelor D, Golant M, Lyman GH, Rhodes N, Yardley D. Chemotherapy-induced alopecia: psychosocial impact and therapeutic approaches. Support Care Cancer. 2004;12:543–549. - PubMed
-
- Fraiser LH, Kanekal S, Kehrer JP. Cyclophosphamide toxicity. Characterising and avoiding the problem. Drugs. 1991;42:781–795. - PubMed
-
- Wang J, Lu Z, Au JL. Protection against chemotherapy-induced alopecia. Pharm Res. 2006;23:2505–2514. - PubMed
-
- Merk HF. Drugs affecting hair growth. Lyne AG, Short BF, editors. Sydney: Augus and Robertson,; Biology of the skin and hair growth. 1965:pp 601–609.
-
- Crounse GR, van Scott EJ. Changes in scalp hair roots as a measure of toxicity from cancer chemotherapeutic drugs. J Invest Dermatol. 1960;35:83–90. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

