Disordered purinergic signaling inhibits pathological angiogenesis in cd39/Entpd1-null mice
- PMID: 17823293
- PMCID: PMC1988887
- DOI: 10.2353/ajpath.2007.070190
Disordered purinergic signaling inhibits pathological angiogenesis in cd39/Entpd1-null mice
Abstract
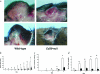
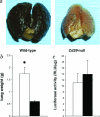
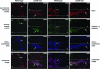
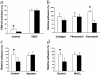
CD39/ecto-nucleoside triphosphate diphosphohydrolase-type-1 (ENTPD1) is the dominant vascular ecto-nucleotidase that catalyzes the phosphohydrolysis of extracellular nucleotides in the blood and extracellular space. This ecto-enzymatic process modulates endothelial cell, leukocyte, and platelet purinergic receptor-mediated responses to extracellular nucleotides in the setting of thrombosis and vascular inflammation. We show here that deletion of Cd39/Entpd1 results in abrogation of angiogenesis, causing decreased growth of implanted tumors and inhibiting development of pulmonary metastases. Qualitative abnormalities of Cd39-null endothelial cell adhesion and integrin dysfunction were demonstrated in vitro. These changes were associated with decreased activation of focal adhesion kinase and extracellular signaling-regulated kinase-1 and -2 in endothelial cells. Our data indicate novel links between CD39/ENTPD1, extracellular nucleotide-mediated signaling, and vascular endothelial cell integrin function that impact on angiogenesis and tumor growth.
Figures

References
-
- Kaczmarek E, Koziak K, Sevigny J, Siegel JB, Anrather J, Beaudoin AR, Bach FH, Robson SC. Identification and characterization of CD39/vascular ATP diphosphohydrolase. J Biol Chem. 1996;271:33116–33122. - PubMed
-
- Enjyoji K, Sevigny J, Lin Y, Frenette PS, Christie PD, Esch JS, Imai M, Edelberg JM, Rayburn H, Lech M, Beeler DL, Csizmadia E, Wagner DD, Robson SC, Rosenberg RD. Targeted disruption of Cd39/ATP diphosphohydrolase results in disordered hemostasis and thromboregulation. Nat Med. 1999;5:1010–1017. - PubMed
-
- Satterwhite CM, Farrelly AM, Bradley ME. Chemotactic, mitogenic, and angiogenic actions of UTP on vascular endothelial cells. Am J Physiol. 1999;45:H1091–H1097. - PubMed
-
- Goepfert C, Sundberg C, Sevigny J, Enjyoji K, Hoshi T, Csizmadia E, Robson SC. Disordered cellular migration and angiogenesis in Cd39-null mice. Circulation. 2001;104:3109–3115. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

