RelB, a new partner of aryl hydrocarbon receptor-mediated transcription
- PMID: 17823304
- PMCID: PMC2346533
- DOI: 10.1210/me.2007-0211
RelB, a new partner of aryl hydrocarbon receptor-mediated transcription
Abstract
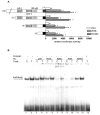
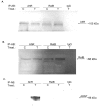
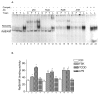
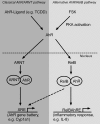
The nuclear factor-kappaB (NF-kappaB) transcription factor family has a crucial role in rapid responses to stress and pathogens. We show that the NF-kappaB subunit RelB is functionally associated with the aryl hydrocarbon receptor (AhR) and mediates transcription of chemokines such as IL-8 via activation of AhR and protein kinase A. RelB physically interacts with AhR and binds to an unrecognized RelB/AhR responsive element of the IL-8 promoter linking two signaling pathways to activate gene transcription. We found a time-dependent recruitment of AhR to the RelB/AhR responsive element site of IL-8 mediated by the AhR ligand 2,3,7,8-tetrachlorodibenzo-p-dioxin (dioxin) and via activation of protein kinase A. Furthermore, NF-kappaB-binding sites that are preferentially recognized by RelB/p52 are a target for RelB/AhR complexes without addition of any stimuli, implicating the endogenous function of the AhR. RelB/AhR complexes are also found to bind on xenobiotic responsive element, and RelB drastically increases the 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced xenobiotic responsive element reporter activity. The interaction of RelB with AhR signaling, and AhR with NF-kappaB RelB signaling pathways represent a new mechanism of cross talk between the two transcription factors.
Figures














References
-
- Chen FE, Huang DB, Chen YQ, Ghosh G. Crystal structure of p50/p65 heterodimer of transcription factor NF-kappaB bound to DNA. Nature. 1998;391:410–403. - PubMed
-
- Dejardin E, Droin NM, Delhase M, Haas E, Cao Y, Makris C, Li ZW, Karin M, Ware CF, Green DR. The lymphotoxin-beta receptor induces different patterns of gene expression via two NF-kappaB pathways. Immunity. 2002;4:525–535. - PubMed
-
- Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004;8:2195–2224. - PubMed

