Intrauterine pulmonary hypertension impairs angiogenesis in vitro: role of vascular endothelial growth factor nitric oxide signaling
- PMID: 17823355
- PMCID: PMC2176095
- DOI: 10.1164/rccm.200705-750OC
Intrauterine pulmonary hypertension impairs angiogenesis in vitro: role of vascular endothelial growth factor nitric oxide signaling
Abstract
Rationale: Mechanisms that impair angiogenesis in neonatal persistent pulmonary hypertension (PPHN) are poorly understood.
Objectives: To determine if PPHN alters fetal pulmonary artery endothelial cell (PAEC) phenotype and impairs growth and angiogenesis in vitro, and if altered vascular endothelial growth factor-nitric oxide (VEGF-NO) signaling contributes to this abnormal phenotype.
Methods: Proximal PAECs were harvested from fetal sheep that had undergone partial ligation of the ductus arteriosus in utero (PPHN) and age-matched control animals. Growth and tube formation +/- VEGF and NO stimulation and inhibition were studied in normal and PPHN PAECs. Western blot analysis was performed for VEGF, VEGF receptor-2 (VEGF-R2), and endothelial NO synthase (eNOS) protein content. NO production with VEGF administration was measured in normal and PPHN PAECs.
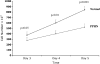
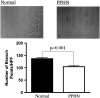
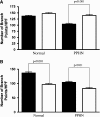
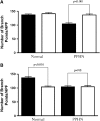
Measurements and main results: PPHN PAECs demonstrate decreased growth and tube formation in vitro. VEGF and eNOS protein expression were decreased in PPHN PAECs, whereas VEGF-R2 protein expression was not different. VEGF and NO increased PPHN PAEC growth and tube formation to values achieved in normal PAECs. VEGF inhibition decreased growth and tube formation in normal and PPHN PAECs. NOS inhibition decreased growth in normal and PPHN PAECs, but tube formation was only reduced in normal PAECs. NO reversed the inhibitory effects of VEGF-R2 inhibition on tube formation in normal and PPHN PAECs. VEGF increased NO production in normal and PPHN PAECs.
Conclusions: PPHN in utero causes sustained impairment of PAEC phenotype in vitro, with reduced PAEC growth and tube formation and down-regulation of VEGF and eNOS protein. VEGF and NO enhanced growth and tube formation of PPHN PAECs.
Figures




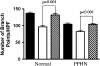

References
-
- Levin DL, Heymann MA, Kitterman JA, Gregory GA, Phibbs RH, Rudolph AM. Persistent pulmonary hypertension of the newborn infant. J Pediatr 1976;89:626–630. - PubMed
-
- Geggel RL, Reid LM. The structural basis of PPHN. Clin Perinatol 1984;11:525–549. - PubMed
-
- Villanueva ME, Zaher FM, Svinarich DM, Konduri G. Decreased gene expression of endothelial nitric oxide synthase in newborns with persistent pulmonary hypertension. Pediatr Res 1998;44:338–343. - PubMed
-
- Villamor E, Le Cras TD, Horan MP, Halbower AC, Tuder RM, Abman SH. Chronic intrauterine pulmonary hypertension impairs endothelial nitric oxide synthase in the ovine fetus. Am J Physiol Lung Cell Mol Physiol 1997;16:L1013–L1020. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

