Haloperidol induces the nuclear translocation of phosphatidylinositol 3'-kinase to disrupt Akt phosphorylation in PC12 cells
- PMID: 17823648
- PMCID: PMC1963350
Haloperidol induces the nuclear translocation of phosphatidylinositol 3'-kinase to disrupt Akt phosphorylation in PC12 cells
Abstract
Objective: The antipsychotic drug haloperidol (HAL) has been linked to apoptosis and to inhibition of prosurvival Akt signalling in pheochromocytoma (PC12) and neuronal cell cultures. However, the mechanism involved is unclear.
Methods: We used HAL to induce cytotoxicity in preneuronal PC12 cells. The expression and the subcellular localization of selected components of the PI3K-Akt survival cascade were monitored with standard biochemical approaches, such as subcellular fractionation, western blot analysis, gene transfer and fluorescence microscopy.
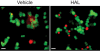
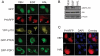
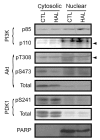
Results: PC12 cell stimulation with the epidermal growth factor (used as a control) results in normal processing of phosphatidylinositol 3'-kinase (PI3K)-Akt signalling (e.g., localization of PI3K to the plasma membrane and phosphorylation of Akt (Ser473). Surprisingly, HAL induces PI3K-generated phosphoinositol [phosphatidylinositol-3,4,5-triphosphate (PIP3), which conflicts with its ability to inhibit Akt. In fact, the production of PIP3s is nuclear, as assessed by the localized concentration of a fluorophore-tagged PIP3-targeting pleckstrin homology protein and a fluorophore-tagged substrate-trapping mutant of the phosphoinositide phosphatase, phosphatase and tensin homologue deleted on chromosome 10 (PTEN). However, phosphoinositide-dependent protein kinase 1 (PDK1, the activating kinase of Akt) does not colocalize to the nucleus with the PI3K complex. This effectively inactivates both cytoplasmic and nuclear pools of Akt.
Conclusion: The differential compartmentalization of effectors of the PI3K-PDK1-Akt pathway is a unique means by which HAL disrupts Akt functioning in PC12 cells.
Objectif: Il existe un lien entre l'halopéridol, un antipsychotique, et l'apoptose et l'inhibition du signal Akt prosurvie dans les phéochromocytomes (PC12) et les cultures de cellules neuronales. Le mécanisme en cause n'est toutefois pas clair.
Méthodes: Nous avons utilisé l'halopéridol pour provoquer la cytotoxicité dans des cellules PC12 à l'état préneuronal. On a surveillé l'expression et la localisation sous-cellulaire d'éléments de la voie de survie PI3K/Akt au moyen de méthodes biochimiques standards comme le fractionnement sous-cellulaire, le transfert de Western, le transfert de gènes et la microscopie par fluorescence.
Résultats: La stimulation des cellules de PC12 au moyen du facteur de croissance épidermique (utilisé comme témoin) provoque un effet normal sur le signal phosphatidylinositol 3'-kinase (PI3K)/Akt (p. ex., localisation de la PI3K sur la membrane plasmatique et phosphorylation de l'Akt [Ser473]). L'halopéridol induit le phosphoïnositol [phosphatidylinositol-3,4,5-triphosphate (PIP3)] généré par la PI3K, ce qui entre en conflit avec sa capacité d'inhiber l'Akt. En fait, la production de PIP3 se fait dans le noyau de la cellule telle qu'évaluée par la concentration localisée, d'une part, d'une protéine homologue à la pleckstrin ciblant PIP3 et marquée par un fluorophore et, d'autre part, d'un mutant de la phosphoïnositide phosphatase, de la phosphatase et du PTEN (phosphatase and tensin homologue deleted on chromosome ten), capable de piéger le substrat et marqué par un fluorophore. La protéine kinase 1 dépendante du phosphoïnositide (PDK1, la kinase activant l'Akt) ne se colocalise toutefois pas dans le noyau avec le complexe PI3K, ce qui inactive en fait l'Akt cytoplasmique et nucléaire.
Conclusion: La compartimentalisation différentielle des effecteurs de la voie de signalisation PI3K-PDK1-Akt est un moyen unique par lequel l'halopéridol perturbe le fonctionnement de l'Akt dans les cellules de PC12.
Keywords: EGF; haloperidol; phospholipids.
Figures
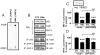

Similar articles
-
Pertussis toxin-sensitive Gi/o proteins are involved in nerve growth factor-induced pro-survival Akt signaling cascade in PC12 cells.Cell Signal. 2005 Jul;17(7):881-90. doi: 10.1016/j.cellsig.2004.11.008. Epub 2004 Dec 15. Cell Signal. 2005. PMID: 15763430
-
Haloperidol disrupts Akt signalling to reveal a phosphorylation-dependent regulation of pro-apoptotic Bcl-XS function.Cell Signal. 2009 Jan;21(1):161-8. doi: 10.1016/j.cellsig.2008.10.005. Epub 2008 Oct 12. Cell Signal. 2009. PMID: 18951975
-
Akt phosphorylation is essential for nuclear translocation and retention in NGF-stimulated PC12 cells.Biochem Biophys Res Commun. 2006 Oct 20;349(2):789-98. doi: 10.1016/j.bbrc.2006.08.120. Epub 2006 Aug 28. Biochem Biophys Res Commun. 2006. PMID: 16956580
-
PTEN, more than the AKT pathway.Carcinogenesis. 2007 Jul;28(7):1379-86. doi: 10.1093/carcin/bgm052. Epub 2007 Mar 6. Carcinogenesis. 2007. PMID: 17341655 Review.
-
Nuclear and mitochondrial signalling Akts in cardiomyocytes.Cardiovasc Res. 2009 May 1;82(2):272-85. doi: 10.1093/cvr/cvp087. Epub 2009 Mar 11. Cardiovasc Res. 2009. PMID: 19279164 Free PMC article. Review.
Cited by
-
Neurodevelopment in schizophrenia: the role of the wnt pathways.Curr Neuropharmacol. 2013 Sep;11(5):535-58. doi: 10.2174/1570159X113119990037. Curr Neuropharmacol. 2013. PMID: 24403877 Free PMC article.
-
The wnt pathway in mood disorders.Curr Neuropharmacol. 2012 Sep;10(3):239-53. doi: 10.2174/157015912803217279. Curr Neuropharmacol. 2012. PMID: 23449817 Free PMC article.
-
An (Immuno) Fluorescence Protocol for Monitoring Monoamine Oxidase A/B Protein Distribution Within the Cell.Methods Mol Biol. 2023;2558:143-161. doi: 10.1007/978-1-0716-2643-6_11. Methods Mol Biol. 2023. PMID: 36169861
-
The PI3K regulatory subunits p55α and p50α regulate cell death in vivo.Cell Death Differ. 2014 Sep;21(9):1442-50. doi: 10.1038/cdd.2014.59. Epub 2014 Jun 6. Cell Death Differ. 2014. PMID: 24902901 Free PMC article.
-
Chronic haloperidol exposure impairs neurodevelopment via Notch1 signaling in human stem cell-derived brain organoids.Sci Rep. 2025 Jul 17;15(1):25945. doi: 10.1038/s41598-025-08855-w. Sci Rep. 2025. PMID: 40676056 Free PMC article.
References
-
- Brunet A, Datta SR, Greenberg ME. Transcription-dependent and independent control of neuronal survival by the PI3K-Akt signaling pathway. Curr Opin Neurobiol 2001;11:297-305. - PubMed
-
- Mora A, Komander D, van Aalten DM, et al. PDK1, the master regulator of AGC kinase signal transduction. Semin Cell Dev Biol 2004; 15:161-70. - PubMed
-
- Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev 1999;13:2905-27. - PubMed
-
- Gericke A, Munson M, Ross AH. Regulation of the PTEN phosphatase. Gene 2006;374:1-9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
