Auditory processing in schizophrenia during the middle latency period (10-50 ms): high-density electrical mapping and source analysis reveal subcortical antecedents to early cortical deficits
- PMID: 17823650
- PMCID: PMC1963354
Auditory processing in schizophrenia during the middle latency period (10-50 ms): high-density electrical mapping and source analysis reveal subcortical antecedents to early cortical deficits
Abstract
Introduction: Auditory sensory processing dysfunction is a core component of schizophrenia, with deficits occurring at 50 ms post-stimulus firmly established in the literature. Given that the initial afference of primary auditory cortex occurs at least 35 ms earlier, however, an essential question remains: how early in sensory processing do such deficits arise, and do they occur during initial cortical afference or earlier, which would implicate subcortical auditory dysfunction.
Objective: To establish the onset of the earliest deficits in auditory processing, we examined the time window demarcating the transition from subcortical to cortical processing: 10 ms to 50 ms during the so-called middle latency responses (MLRs). These remain to be adequately characterized in patients with schizophrenia.
Methods: We recorded auditory evoked potentials (AEPs) to simple tone-pips from 15 control subjects and 21 medicated patients with longer-term schizophrenia or schizoaffective disorder (illness duration 16 yr, standard deviation [SD] 9.4 yr), using high-density electrical scalp recordings. Between-group analyses assessed the integrity of the MLRs across groups. In addition, 2 source-localization models were conducted to address whether a distinction between subcortical and cortical generators of the MLRs can be made and whether evidence for differential dorsal and ventral pathway contributions to auditory processing deficits can be established.
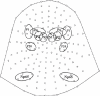
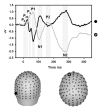
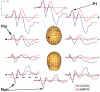
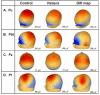
Results: Robust auditory processing deficits were found for patients as early as 15 ms. Evidence for subcortical generators of the earliest MLR component (P20) was provided by source analysis. Topographical mapping and source localization also pointed to greater decrements in processing in the dorsal auditory pathway of patients, providing support for a theory of pervasive deficits that are organized along the lines of a dorsal-ventral distinction.
Conclusions: Auditory sensory dysfunction in schizophrenia begins extremely early in processing, is evident during initial cortical afference and is also seen at earlier subcortical processing stages in the thalamus. The implication is that well-established sensory processing deficits in schizophrenia may be secondary to earlier subcortical dysfunction. Our findings do not preclude the possibility of even earlier deficits in auditory sensory processing during the auditory brainstem responses.
Introduction: Le dysfonctionnement du traitement sensoriel auditif est un élément fondamental de la schizophrénie, et les déficits qui se produisent 50 ms après le stimulus sont fermement établis dans les écrits. Cependant, comme l'afférence initiale du cortex auditif primaire se produit au moins 35 ms plus tôt, une question essentielle persiste : à quel stade du début du traitement sensoriel ces déficits surgissent-ils, et se produisent-ils au cours de l'afférence corticale initiale ou plus tôt, ce qui mettrait en cause un dysfonctionnement auditif infracortical?
Objectif: Pour déterminer l'apparition des déficits les plus précoces dans le traitement auditif, nous avons analysé le créneau temporel qui démarque la transition du traitement infracortical au traitement cortical, soit le créneau de 10 à 50 ms au cours des réponses dites à latence intermédiaire (RLI). Il reste à les caractériser adéquatement chez les patients atteints de schizophrénie.
Méthodes: Nous avons enregistré les potentiels évoqués auditifs (PEA) par de simples tonalités chez 15 sujets témoins et chez 21 patients prenant des médicaments et atteints de schizophrénie de longue durée ou de troubles schizo-affectifs (durée de la maladie, 16 ans; écart-type [ET], 9,4 ans) en utilisant des enregistrements électriques à haute densité sur le cuir chevelu. Des analyses entre groupes ont permis de déterminer l'intégrité des RLI entre les groupes. En outre, on a exécuté deux modèles de localisation de la source afin de déterminer s'il est possible d'établir une distinction entre les générateurs infracorticaux et corticaux des RLI et s'il est possible de démontrer que les voies dorsale et ventrale contribuent différemment au déficit du traitement auditif.
Résultats: On a constaté des déficits solides du traitement auditif chez les patients dès 15 ms. L'analyse de la source a produit des preuves dans le cas des générateurs infracorticaux de la composante la plus précoce des RLI (P20). L'analyse topographique et la localisation de la source ont aussi indiqué que les décréments étaient plus importants dans le traitement dans la voie auditive dorsale des patients, ce qui appuie une théorie relative au déficit omniprésent structuré suivant les lignes de démarcation d'une distinction dorsale-ventrale.
Conclusions: Le dysfonctionnement sensoriel auditif dans les cas de schizophrénie commence extrêmement tôt au cours du traitement de l'information, se manifeste au cours de l'afférence corticale initiale et on le constate aussi à des stades plus précoces du traitement infracortical dans le thalamus. Il en découle que des déficits bien établis du traitement sensoriel dans les cas de schizophrénie peuvent être secondaires à un dysfonctionnement infracortical antérieur. Nos constatations n'excluent pas la possibilité de déficits survenant encore plus tôt dans le traitement sensoriel auditif, au cours des réponses du cervelet au stimulus auditif.
Keywords: auditory-evoked potentials; electroencephalography; event-related potentials.
Figures




References
-
- Butler PD, Schechter I, Zemon V, et al. Dysfunction of early-stage visual processing in schizophrenia. Am J Psychiatry 2001;158:1126-33. - PubMed
-
- Foxe JJ, Doniger GM, Javitt DC. Early visual processing deficits in schizophrenia: impaired P1 generation revealed by high-density electrical mapping. Neuroreport 2001;12:3815-20. - PubMed
-
- Buchsbaum MS, Awsare SV, Holcomb HH, et al. Topographic differences between normals and schizophrenics: the N120 evoked potential component. Neuropsychobiology 1986;15:1-6. - PubMed
-
- Javitt DC, Doneshka P, Grochowski S, et al. Impaired mismatch negativity generation reflects widespread dysfunction of working memory in schizophrenia. Arch Gen Psychiatry 1995;52:550-8. - PubMed
-
- Javitt DC, Strous RD, Grochowski S, et al. Impaired precision, but normal retention, of auditory sensory (“echoic”) memory information in schizophrenia. J Abnorm Psychol 1997;106:315-24. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
