The allosteric modulation of lipases and its possible biological relevance
- PMID: 17825093
- PMCID: PMC2020465
- DOI: 10.1186/1742-4682-4-34
The allosteric modulation of lipases and its possible biological relevance
Abstract
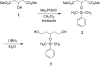
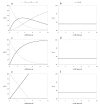
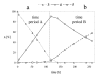
Background: During the development of an enantioselective synthesis using the lipase from Mucor miehei an unusual reaction course was observed, which was analyzed precisely. For the first time an allosteric modulation of a lipase changing its selectivity was shown.
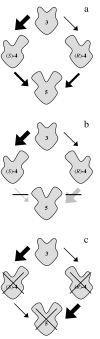
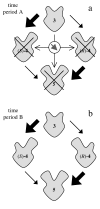
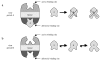
Theory: Considering the biological relevance of the discovered regulation mechanism we developed a theory that describes the regulation of energy homeostasis and fat metabolism.
Conclusion: This theory represents a new approach to explain the cause of the metabolic syndrome and provides an innovative basis for further research activity.
Figures







Similar articles
-
Lipase genes in Mucor circinelloides: identification, sub-cellular location, phylogenetic analysis and expression profiling during growth and lipid accumulation.J Ind Microbiol Biotechnol. 2016 Oct;43(10):1467-80. doi: 10.1007/s10295-016-1820-0. Epub 2016 Aug 17. J Ind Microbiol Biotechnol. 2016. PMID: 27535142
-
Burkholderia cepacia lipase: A versatile catalyst in synthesis reactions.Biotechnol Bioeng. 2018 Jan;115(1):6-24. doi: 10.1002/bit.26458. Epub 2017 Oct 30. Biotechnol Bioeng. 2018. PMID: 28941272 Review.
-
Esterification activity and conformation studies of Burkholderia cepacia lipase in conventional organic solvents, ionic liquids and their co-solvent mixture media.Bioresour Technol. 2010 Dec;101(24):9822-4. doi: 10.1016/j.biortech.2010.07.107. Epub 2010 Aug 1. Bioresour Technol. 2010. PMID: 20713309
-
Insights into lid movements of Burkholderia cepacia lipase inferred from molecular dynamics simulations.Proteins. 2009 Nov 15;77(3):509-23. doi: 10.1002/prot.22462. Proteins. 2009. PMID: 19475702
-
Creation of novel enantioselective lipases by SIMPLEX.Methods Mol Biol. 2007;375:165-81. doi: 10.1007/978-1-59745-388-2_9. Methods Mol Biol. 2007. PMID: 17634602 Review.
Cited by
-
Beauveria bassiana Lipase A expressed in Komagataella (Pichia) pastoris with potential for biodiesel catalysis.Front Microbiol. 2015 Oct 7;6:1083. doi: 10.3389/fmicb.2015.01083. eCollection 2015. Front Microbiol. 2015. PMID: 26500628 Free PMC article.
-
Regioselective Deacetylation in Nucleosides and Derivatives.Chembiochem. 2024 Dec 2;25(23):e202400360. doi: 10.1002/cbic.202400360. Epub 2024 Sep 5. Chembiochem. 2024. PMID: 39037890 Free PMC article. Review.
References
-
- Ghanem A. Trends in lipase-catalyzed asymmetric access to enantiomerically pure/enriched compounds. Tetrahedron. 2007;63:1721–1754. doi: 10.1016/j.tet.2006.09.110. - DOI
-
- Bornscheuer UT, Kazlauskas RJ. Hydrolases in Organic Synthesis. Wiley-VCH: Weinheim Germany; 1999.
-
- Cambillau C, van Tilbeurgh H. Structure of hydrolases: lipases and cellulases. Curr Opin Struc Biol. 1993;3:885–895. doi: 10.1016/0959-440X(93)90152-B. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

