Regulation of CYP3A4 and CYP2B6 expression by liver X receptor agonists
- PMID: 17825266
- PMCID: PMC2080783
- DOI: 10.1016/j.bcp.2007.07.040
Regulation of CYP3A4 and CYP2B6 expression by liver X receptor agonists
Abstract
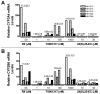
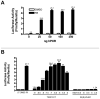
The liver X receptor (LXR) agonists, 24(S),25-epoxycholesterol and T0901317, were previously shown to be capable of inducing CYP3A expression in primary cultured rodent hepatocytes through activation of the pregnane X receptor (PXR). In this study, the abilities of these two LXR agonists to regulate CYP3A4 and CYP2B6 mRNA expression in primary cultures of human hepatocytes were evaluated. Treatment with 10 or 30 microM of the endogenous oxysterol, 24(S),25-epoxycholesterol, had no effect on CYP3A4 mRNA content in five preparations of primary cultured human hepatocytes, while 30 microM 24(S),25-epoxycholesterol treatment increased CYP2B6 mRNA content by approximately two-fold. By comparison, treatment with the synthetic LXR agonist, T0901317, potently increased CYP3A4 and CYP2B6 mRNA levels in the human hepatocyte cultures, producing multi-fold increases at 10nM. Using a HepG2-based transactivation assay, T0901317 activated human PXR with an EC(50) approximately 20nM, which was more than 10-fold lower than that of the potent PXR ligand, SR-12813, while treatment with 24(S),25-epoxycholesterol failed to induce reporter expression in this assay. Therefore, while 24(S),25-epoxycholesterol-mediated PXR activation and CYP3A induction does not appear to be conserved from rodent to human, T0901317 is among the most potent known activators of human PXR.
Figures

References
-
- Tontonoz P, Mangelsdorf DJ. Liver X receptor signaling pathways in cardiovascular disease. Mol Endocrinol. 2003;17:985–993. - PubMed
-
- Spencer TA, Li D, Russel JS, Collins JL, Bledsoe RK, Consler TG, Moore LB, Galardi CM, McKee DD, Moore JT, Watson MA, Parks DJ, Lambert MH, Willson TM. Pharmacophore analysis of the nuclear oxysterol receptor LXRα. J Med Chem. 2001;44:886–897. - PubMed
-
- Nelson JA, Steckbeck SR, Spencer TA. Biosynthesis of 24,25-epoxycholesterol from squalene 2,3:22,23-dioxide. J Biol Chem. 1981;256:1067–1068. - PubMed
-
- Zhang Z, Li D, Blanchard DE, Lear SR, Erickson SK, Spencer TA. Key regulatory oxysterols in liver: Analysis as d4-3-ketone derivatives by HPLC and response to physiological perturbations. J Lipid Res. 2001;42:649–658. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

