Use of Z310 cells as an in vitro blood-cerebrospinal fluid barrier model: tight junction proteins and transport properties
- PMID: 17825520
- PMCID: PMC2677988
- DOI: 10.1016/j.tiv.2007.07.007
Use of Z310 cells as an in vitro blood-cerebrospinal fluid barrier model: tight junction proteins and transport properties
Abstract
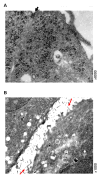
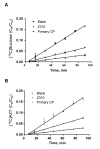
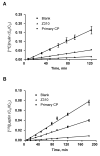
Immortalized rat choroidal epithelial Z310 cells have the potential to become an in vitro model for studying transport of materials at blood-cerebrospinal fluid barrier (BCB) (Shi and Zheng, 2005) [Shi, L.Z., Zheng, W., 2005. Establishment of an in vitro brain barrier epithelial transport system for pharmacological and toxicological study. Brain Research 1057, 37-48]. This study was designed to demonstrate the presence of tight junction properties in Z310 cells and the functionality of Z310 monolayer in transport of selected model compounds. Western blot analyses revealed the presence of claudin-1, ZO-1, and occludin in Z310 cells. Transmission electron microscopy showed a "tight junction" type of structure in the sub-apical lateral membranes between adjacent Z310 cells. Real-time RT-PCR revealed that Z310 cells expressed representative transporters such as DMT1, MTP1, TfR, p-glycoprotein, ATP7A, ZnT1, ABCC1, Oat3, OCT1 and OB-Ra. Moreover, Z310 cells cultured in a two-chamber Transwell device possessed the ability to transport zidovudine (anionic drug), thyroxine (hormone), thymidine (nucleoside), and leptin (large polypeptide) with kinetic properties similar to those obtained from the in vitro model based on primary culture of choroidal epithelial cells. Taken together, these data indicate that the Z310 BCB model expresses major tight junction proteins and forms a tight barrier in vitro. The model also exhibits the ability to transport substances of various categories across the barrier.
Figures


References
-
- Bjorbaek C, Elmquist JK, Michl P, Ahima RS, van Bueren A, McCall AL, Flier JS. Expression of leptin receptor isoforms in rat brain microvessels. Endocrinology. 1998;139:3485–3491. - PubMed
-
- Dehouck MP, Jolliet-Riant P, Bree F, Fruchart JC, Cecchelli R, Tillement JP. Drug transfer across the blood– brain barrier: correlation between in vitro and in vivo models. Journal of Neurochemistry. 1992;58:1790–1797. - PubMed
-
- Dratman MB, Crutchfield FL, Schoenhoff MB. Transport of iodothyronines from bloodstream to brain: contributions by blood:brain and choroid plexus:cerebrospinal fluid barriers. Brain Research. 1991;554:229–236. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

