Disruption of intraflagellar transport in adult mice leads to obesity and slow-onset cystic kidney disease
- PMID: 17825558
- PMCID: PMC2084209
- DOI: 10.1016/j.cub.2007.08.034
Disruption of intraflagellar transport in adult mice leads to obesity and slow-onset cystic kidney disease
Abstract
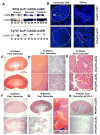
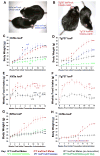
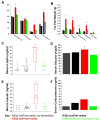
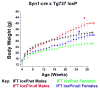
The assembly of primary cilia is dependent on intraflagellar transport (IFT), which mediates the bidirectional movement of proteins between the base and tip of the cilium. In mice, congenic mutations disrupting genes required for IFT (e.g., Tg737 or the IFT kinesin Kif3a) are embryonic lethal, whereas kidney-specific disruption of IFT results in severe, rapidly progressing cystic pathology. Although the function of primary cilia in most tissues is unknown, in the kidney they are mechanosenstive organelles that detect fluid flow through the tubule lumen. The loss of this flow-induced signaling pathway is thought to be a major contributing factor to cyst formation. Recent data also suggest that there is a connection between ciliary dysfunction and obesity as evidenced by the discovery that proteins associated with human obesity syndromes such as Alström and Bardet-Biedl localize to this organelle. To more directly assess the importance of cilia in postnatal life, we utilized conditional alleles of two ciliogenic genes (Tg737 and Kif3a) to systemically induce cilia loss in adults. Surprisingly, the cystic kidney pathology in these mutants is dependent on the time at which cilia loss was induced, suggesting that cyst formation is not simply caused by impaired mechanosensation. In addition to the cystic pathology, the conditional cilia mutant mice become obese, are hyperphagic, and have elevated levels of serum insulin, glucose, and leptin. We further defined where in the body cilia are required for normal energy homeostasis by disrupting cilia on neurons throughout the central nervous system and on pro-opiomelanocortin-expressing cells in the hypothalamus, both of which resulted in obesity. These data establish that neuronal cilia function in a pathway regulating satiety responses.
Figures
References
-
- Murcia NS, Richards WG, Yoder BK, Mucenski ML, Dunlap JR, Woychik RP. The Oak Ridge Polycystic Kidney (orpk) disease gene is required for left-right axis determination. Development. 2000;127:2347–2355. - PubMed
-
- Singla V, Reiter JF. The primary cilium as the cell's antenna: signaling at a sensory organelle. Science. 2006;313:629–633. - PubMed
-
- Praetorius HA, Spring KR. The renal cell primary cilium functions as a flow sensor. Curr Opin Nephrol Hypertens. 2003;12:517–520. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 NS047466/NS/NINDS NIH HHS/United States
- P30 NS057098/NS/NINDS NIH HHS/United States
- P30NS057098/NS/NINDS NIH HHS/United States
- R01DK65655/DK/NIDDK NIH HHS/United States
- R01 DK058382/DK/NIDDK NIH HHS/United States
- R01 DK065655/DK/NIDDK NIH HHS/United States
- P30 CA13148/CA/NCI NIH HHS/United States
- R56 DK075996/DK/NIDDK NIH HHS/United States
- P30 DK056336/DK/NIDDK NIH HHS/United States
- P30 DK074038/DK/NIDDK NIH HHS/United States
- P30 NS47466/NS/NINDS NIH HHS/United States
- R56DK075996/DK/NIDDK NIH HHS/United States
- P30 CA013148/CA/NCI NIH HHS/United States
- R01 DK075996/DK/NIDDK NIH HHS/United States
- P30DK56336/DK/NIDDK NIH HHS/United States
- DK58382/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

