A robust immunoassay for anti-interferon autoantibodies that is highly specific for patients with autoimmune polyglandular syndrome type 1
- PMID: 17825626
- PMCID: PMC2080870
- DOI: 10.1016/j.clim.2007.07.015
A robust immunoassay for anti-interferon autoantibodies that is highly specific for patients with autoimmune polyglandular syndrome type 1
Abstract
High titer antibodies to type 1 interferons have been recently reported as being highly specific for patients with autoimmune polyglandular syndrome type 1 (APS1) in Finnish and Norwegian patients with mutations in the AIRE gene. Those studies employed a complex neutralization assay to define the type 1 interferon autoantibodies. Here we have established a competitive europium time resolved fluorescence assay for IFN-alpha autoantibodies and measured sera from subjects with APS1, first degree relatives of APS1 patients, patients with Addison's disease or Type 1 diabetes. The europium-based immunoassay utilizes plate bound human IFN-alpha incubated with sera with or without competition with fluid phase IFN-alpha, followed by anti-IgG biotinylated antibody and detection with streptavidin-europium. The index of IFN-alpha Ab was calculated as (CPS (Counts per second) without competition-CPS with competition)/(CPS positive standard sera without competition-CPS positive standard sera with competition). RESULTS are reported for raw CPS and indices and are compared across the different subjects.
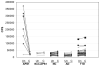
Results: For normal controls (n=100) CPS without competition were 31,237+/-17,328 CPS while after subtracting the competition value, the results were -6563+/-10,303 CPS. The initial APS1 patient (used to create the index as 1.0) gave 394,063 CPS without competition and a delta of 363,662+/-31,587 CPS with competition. Scatchard plot analysis of this patient sample revealed a high avidity for IFN-alpha (K(d) of 0.5 nM). The CPS, delta, and index for 6/7 APS1 patients were strongly positive and 3 standard deviations or more above that of the normal controls. Using a cut-off of 2 standard deviations above normal controls, relatives of APS1 patients were negative for type I interferon autoantibodies as were 71 patients with Addison's disease (non-APS1) and 141 Type 1 diabetes patients. This simple high throughput competitive europium time resolved fluorescence assay had a sensitivity of > or =86% or greater and a specificity of >99.5%.
Figures



References
-
- Perheentupa J. Autoimmune Polyendocrinopathy-Candidiasis-Ectodermal Dystrophy. J Clin Endocrinol Metab. 2006;91:2843–2850. - PubMed
-
- Nagamine K, Peterson P, Scott HS, Kudoh J, Minoshima S, Heino M, Krohn KJ, Lalioti MD, Mullis PE, Antonarakis SE, Kawasaki K, Asakawa S, Ito F, Shimizu N. Positional cloning of the APECED gene. Nat Genet. 1997;17:393–8. - PubMed
-
- An autoimmune disease, APECED, caused by mutations in a novel gene featuring two PHD-type zinc-finger domains. The Finnish-German APECED Consortium. Autoimmune Polyendocrinopathy-Candidiasis-Ectodermal Dystrophy. Nat Genet. 1997;17:399–403. - PubMed
-
- Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, von Boehmer H, Bronson R, Dierich A, Benoist C, Mathis D. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298:1395–401. - PubMed
-
- Liston A, Lesage S, Wilson J, Peltonen L, Goodnow CC. Aire regulates negative selection of organ-specific T cells. Nat Immunol. 2003;4:350–4. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

