Role of retinol in protecting epithelial cell damage induced by Clostridium difficile toxin A
- PMID: 17825865
- PMCID: PMC2268866
- DOI: 10.1016/j.toxicon.2007.07.010
Role of retinol in protecting epithelial cell damage induced by Clostridium difficile toxin A
Abstract
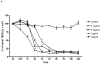
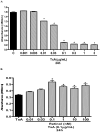
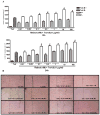
Vitamin A (retinol), a fat-soluble vitamin, is an essential nutrient for the normal functioning of the visual system, epithelial cell integrity and growth, immunity, and reproduction. Our group has investigated the effect of high doses of oral vitamin A on early childhood diarrhea in our prospective community-based studies from Northeast Brazil and found a beneficial role in reducing the mean duration but not incidence of diarrheal episodes. In this study, we explored the role of retinol supplementation in intestinal cell lines following Clostridium difficile toxin A (TxA) challenge. C. difficile is the most common anaerobic pathogen borne with antibiotic-borne diarrhea and pseudomembranous colitis. Since retinol is critical for the integrity of tight junctions and to modulate the cell cycle, we have focused on changes in transepithelial electrical resistance (TEER) in Caco-2, a more differentiated intestinal cell line, and on models of cell proliferation, migration and viability in IEC-6 cells, an undifferentiated crypt cell line, following TxA injury. In this model, retinol therapy reduced apoptosis, improved cell migration and proliferation, and prevented the reduction in TEER, following C. difficile TxA challenge in a glutamine-free medium. These results suggest the role of retinol in protecting intestinal epithelial barrier function from C. difficile TxA enterotoxic damage.
Figures




References
-
- Baltes S, Nau H, Lampen A. All-trans retinoic acid enhances differentiation and influences permeability of intestinal Caco-2 cells under serum-free conditions. Dev Growth Differ. 2004;46(6):503–514. - PubMed
-
- Barreto ML, Santos LM, Assis AM, Araujo MP, Farenzena GG, Santos PA, Fiaccone RL. Effect of vitamin A supplementation on diarrhoea and acute lower-respiratory-tract infections in young children in Brazil. Lancet. 1994;344(8917):228–231. - PubMed
-
- Bartlett JG, Taylor NS, Chang T, Dzink J. Clinical and laboratory observations in Clostridium difficile colitis. Am J Clin Nutr. 1980;33 11:2521–2526. - PubMed
-
- Beyzadeoglu M, Balkan M, Demiriz M, Tibet H, Dirican B, Oner K, Pak Y. Protective effect of vitamin A on acute radiation injury in the small intestine. Radiat Med. 1997;15(1):1–5. - PubMed
-
- Blomhoff R, Blomhoff HK. Overview of retinoid metabolism and function. J Neurobiol. 2006;66(7):606–630. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

