Discovery of platelet-type 12-human lipoxygenase selective inhibitors by high-throughput screening of structurally diverse libraries
- PMID: 17826100
- PMCID: PMC2203963
- DOI: 10.1016/j.bmc.2007.08.015
Discovery of platelet-type 12-human lipoxygenase selective inhibitors by high-throughput screening of structurally diverse libraries
Abstract
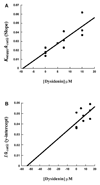
Human lipoxygenases (hLO) have been implicated in a variety of diseases and cancers and each hLO isozyme appears to have distinct roles in cellular biology. This fact emphasizes the need for discovering selective hLO inhibitors for both understanding the role of specific lipoxygenases in the cell and developing pharmaceutical therapeutics. To this end, we have modified a known lipoxygenase assay for high-throughput (HTP) screening of both the National Cancer Institute (NCI) and the UC Santa Cruz marine extract library (UCSC-MEL) in search of platelet-type 12-hLO (12-hLO) selective inhibitors. The HTP screen led to the characterization of five novel 12-hLO inhibitors from the NCI repository. One is the potent but non-selective michellamine B, a natural product, anti-viral agent. The other four compounds were selective inhibitors against 12-hLO, with three being synthetic compounds and one being alpha-mangostin, a natural product, caspase-3 pathway inhibitor. In addition, a selective inhibitor was isolated from the UCSC-MEL (neodysidenin), which has a unique chemical scaffold for a hLO inhibitor. Due to the unique structure of neodysidenin, steady-state inhibition kinetics were performed and its mode of inhibition against 12-hLO was determined to be competitive (K(i)=17microM) and selective over reticulocyte 15-hLO-1 (K(i) 15-hLO-1/12-hLO>30).
Figures


Similar articles
-
Structure-activity relationship studies of flavonoids as potent inhibitors of human platelet 12-hLO, reticulocyte 15-hLO-1, and prostate epithelial 15-hLO-2.Bioorg Med Chem. 2007 Dec 1;15(23):7408-25. doi: 10.1016/j.bmc.2007.07.036. Epub 2007 Aug 22. Bioorg Med Chem. 2007. PMID: 17869117 Free PMC article.
-
Novel human lipoxygenase inhibitors discovered using virtual screening with homology models.J Med Chem. 2006 Feb 23;49(4):1356-63. doi: 10.1021/jm050639j. J Med Chem. 2006. PMID: 16480270
-
Discovery of potent and selective inhibitors of human reticulocyte 15-lipoxygenase-1.J Med Chem. 2010 Oct 28;53(20):7392-404. doi: 10.1021/jm1008852. J Med Chem. 2010. PMID: 20866075 Free PMC article.
-
Selective Small Molecule Inhibitors of 12-Human Lipoxygenase (12-hLO).2009 Nov 30 [updated 2011 Mar 25]. In: Probe Reports from the NIH Molecular Libraries Program [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2010–. 2009 Nov 30 [updated 2011 Mar 25]. In: Probe Reports from the NIH Molecular Libraries Program [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2010–. PMID: 21735604 Free Books & Documents. Review.
-
Current progress in natural product-like libraries for discovery screening.Comb Chem High Throughput Screen. 2004 Nov;7(7):631-43. doi: 10.2174/1386207043328418. Comb Chem High Throughput Screen. 2004. PMID: 15578925 Review.
Cited by
-
Pseudoperoxidase investigations of hydroperoxides and inhibitors with human lipoxygenases.Bioorg Med Chem. 2013 Jul 1;21(13):3894-9. doi: 10.1016/j.bmc.2013.04.016. Epub 2013 Apr 18. Bioorg Med Chem. 2013. PMID: 23669189 Free PMC article.
-
Isotope sensitive branching and kinetic isotope effects in the reaction of deuterated arachidonic acids with human 12- and 15-lipoxygenases.Biochemistry. 2008 Jul 8;47(27):7295-303. doi: 10.1021/bi800308q. Epub 2008 Jun 12. Biochemistry. 2008. PMID: 18547056 Free PMC article.
-
12-lipoxygenase: a potential target for novel anti-platelet therapeutics.Cardiovasc Hematol Agents Med Chem. 2011 Jul 1;9(3):154-64. doi: 10.2174/187152511797037619. Cardiovasc Hematol Agents Med Chem. 2011. PMID: 21838667 Free PMC article. Review.
-
Screening for Small Molecule Inhibitors of BMP-Induced Osteoblastic Differentiation from Indonesian Marine Invertebrates.Mar Drugs. 2020 Nov 30;18(12):606. doi: 10.3390/md18120606. Mar Drugs. 2020. PMID: 33265937 Free PMC article.
-
Substrate specificity changes for human reticulocyte and epithelial 15-lipoxygenases reveal allosteric product regulation.Biochemistry. 2008 Jul 15;47(28):7364-75. doi: 10.1021/bi800550n. Epub 2008 Jun 21. Biochemistry. 2008. PMID: 18570379 Free PMC article.
References
-
- Solomon EI, Zhou J, Neese F, Pavel EG. Chem. Biol. 1997;4:795. - PubMed
-
- Samuelsson B, Dahlen SE, Lindgren JA, Rouzer CA, Serhan CN. Science. 1987;237:1171. - PubMed
-
- Ford-Hutchinson AW, Gresser M, Young RN. Annu. Rev. Biochem. 1994;63:383. - PubMed
-
- Hussain H, Shornick LP, Shannon VR, Wilson JD, Funk CD, Pentland AP, Holtzman MJ. Am. J. Physiol. Cell Physiol. 1994;266:C243. - PubMed
-
- Kamitani H, Geller M, Eling TJ. Biol. Chem. 1998;273:21569. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Research Materials

