Pathology of atherosclerosis and stenting
- PMID: 17826632
- PMCID: PMC2704337
- DOI: 10.1016/j.nic.2007.03.006
Pathology of atherosclerosis and stenting
Abstract
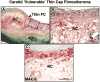
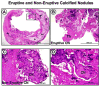
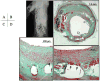
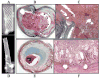
Atherosclerotic plaque at the carotid bifurcation is the primary cause of ischemic strokes and the degree of carotid stenosis is strongly associated with stroke risk in symptomatic patients. Stroke is the third-leading cause of death in the United States, constituting approximately 700,000 cases each year. In this article, the authors discuss the natural history of carotid and intracranial atherosclerosis, based on their broader knowledge of coronary atherosclerosis. Early to more advanced progressive lesions of the carotid are categorized, based on descriptive morphologic events originally cited for the coronary circulation. The histologic features associated with symptomatic and asymptomatic carotid disease are also addressed, along with the issues surrounding current stent-based therapies for the prevention of major recurrent vascular events.
Figures






References
-
- North American Symptomatic Carotid Endarterectomy Trial Collaborators N. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991;325:445–453. - PubMed
-
- Thom T, Haase N, Rosamond W, Howard VJ, Rumsfeld J, Manolio T, Zheng ZJ, Flegal K, O'Donnell C, Kittner S, Lloyd-Jones D, Goff DC, Jr, Hong Y, Adams R, Friday G, Furie K, Gorelick P, Kissela B, Marler J, Meigs J, Roger V, Sidney S, Sorlie P, Steinberger J, Wasserthiel-Smoller S, Wilson M, Wolf P. Heart disease and stroke statistics--2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113:e85–151. - PubMed
-
- Carr S, Farb A, Pearce WH, Virmani R, Yao JS. Atherosclerotic plaque rupture in symptomatic carotid artery stenosis. J Vasc Surg. 1996;23:755–765. discussion 765–756. - PubMed
-
- Spagnoli LG, Mauriello A, Sangiorgi G, Fratoni S, Bonanno E, Schwartz RS, Piepgras DG, Pistolese R, Ippoliti A, Holmes DR., Jr Extracranial thrombotically active carotid plaque as a risk factor for ischemic stroke. Jama. 2004;292:1845–1852. - PubMed
-
- Gronholdt ML, Nordestgaard BG, Schroeder TV, Vorstrup S, Sillesen H. Ultrasonic echolucent carotid plaques predict future strokes. Circulation. 2001;104:68–73. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

