Small molecules affecting transcription in Friedreich ataxia
- PMID: 17826840
- PMCID: PMC2080619
- DOI: 10.1016/j.pharmthera.2007.06.014
Small molecules affecting transcription in Friedreich ataxia
Abstract
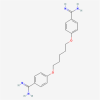
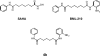
This review concerns the development of small molecule therapeutics for the inherited neurodegenerative disease Friedreich ataxia (FRDA). FRDA is caused by transcriptional repression of the nuclear FXN gene, encoding the essential mitochondrial protein frataxin and accompanying loss of frataxin protein. Frataxin insufficiency leads to mitochrondrial dysfunction and progressive neurodegeneration, along with scoliosis, diabetes and cardiomyopathy. Individuals with FRDA generally die in early adulthood from the associated heart disease, the most common cause of death in FRDA. While antioxidants and iron chelators have shown promise in ameliorating the symptoms of the disease, there is no effective therapy for FRDA that addresses the cause of the disease, the loss of frataxin protein. Gene therapy and protein replacement strategies for FRDA are promising approaches; however, current technology is not sufficiently advanced to envisage treatments for FRDA coming from these approaches in the near future. Since the FXN mutation in FRDA, expanded GAA.TTC triplets in an intron, does not alter the amino acid sequence of frataxin protein, gene reactivation would be of therapeutic benefit. Thus, a number of laboratories have focused on small molecule activators of FXN gene expression as potential therapeutics, and this review summarizes the current status of these efforts, as well as the molecular basis for gene silencing in FRDA.
Figures


References
-
- Al-Mahdawi S, Pinto RM, Varshney D, Lawrence L, Lowrie MB, Hughes S, Webster Z, Blake J, Cooper JM, King R, Pook MA. GAA repeat expansion mutation mouse models of Friedreich ataxia exhibit oxidative stress leading to progressive neuronal and cardiac pathology. Genomics. 2006;88:580–590. - PMC - PubMed
-
- Annunziato AT, Frado LL, Seale RL, Woodcock CL. Treatment with sodium butyrate inhibits the complete condensation of interphase chromatin. Chromosoma. 1988;96:132–138. - PubMed
-
- Babcock M, de Silva D, Oaks R, Davis-Kaplan S, Jiralerspong S, Montermini L, Pandolfo M, Kaplan J. Regulation of mitochondrial iron accumulation by Yfh1p, a putative homolog of frataxin. Science. 1997;276:1709–1712. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

