Conformationally constrained analogues of 2-arachidonoylglycerol
- PMID: 17826996
- PMCID: PMC3679891
- DOI: 10.1016/j.bmcl.2007.07.064
Conformationally constrained analogues of 2-arachidonoylglycerol
Abstract
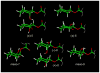
Novel monocyclic analogues of 2-arachidonoylglycerol (2-AG) were designed in order to explore the pharmacophoric conformations of this endocannabinoid ligand at the key cannabinergic proteins. All 2-arachidonoyl esters of 1,2,3-cyclohexanetriol [meso-7 (AM5504), (+/-)-8 (AM5503), and meso-9 (AM5505)] were synthesized by regioselective acylation of 2,3-dihydroxycyclohexanone followed by selective reductions. The optically active isomers (+)-8 (AM4434) and (-)-8 (AM4435) were synthesized from (2S,3S)- and (2R,3R)-2,3-dihydroxycyclohexanone, respectively, via a chemoenzymatic route. These head group constrained and conformationally restricted analogues of 2-AG as well as the 1-keto precursors were evaluated as substrates for the endocannabinoid deactivating hydrolytic enzymes monoacylglycerol lipase (MGL) and fatty acid amide hydrolase (FAAH), and also were tested for their affinities for CB1 and CB2 cannabinoid receptors. The observed biochemical differences between these ligands can help define the conformational requirements for 2-AG activity at each of the above endocannabinoid protein targets.
Figures


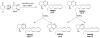

Similar articles
-
Endocannabinoid binding to the cannabinoid receptors: what is known and what remains unknown.Curr Med Chem. 2010;17(14):1468-86. doi: 10.2174/092986710790980005. Curr Med Chem. 2010. PMID: 20166921 Free PMC article. Review.
-
1-, 2- and 3-AG as substrates of the endocannabinoid enzymes and endogenous ligands of the cannabinoid receptor 1.Biochem Biophys Res Commun. 2022 Feb 5;591:31-36. doi: 10.1016/j.bbrc.2021.12.105. Epub 2021 Dec 28. Biochem Biophys Res Commun. 2022. PMID: 34995983
-
Inhibition of monoacylglycerol lipase and fatty acid amide hydrolase by analogues of 2-arachidonoylglycerol.Br J Pharmacol. 2004 Nov;143(6):774-84. doi: 10.1038/sj.bjp.0705948. Epub 2004 Oct 18. Br J Pharmacol. 2004. PMID: 15492019 Free PMC article.
-
Alterations in endocannabinoid tone following chemotherapy-induced peripheral neuropathy: effects of endocannabinoid deactivation inhibitors targeting fatty-acid amide hydrolase and monoacylglycerol lipase in comparison to reference analgesics following cisplatin treatment.Pharmacol Res. 2013 Jan;67(1):94-109. doi: 10.1016/j.phrs.2012.10.013. Epub 2012 Nov 2. Pharmacol Res. 2013. PMID: 23127915 Free PMC article.
-
Cannabinergic ligands.Chem Phys Lipids. 2002 Dec 31;121(1-2):3-19. doi: 10.1016/s0009-3084(02)00143-3. Chem Phys Lipids. 2002. PMID: 12505686 Review.
Cited by
-
Chiral Me-2-arachidonoyl Glycerols: The First Potent Endocannabinoid Glyceride Templates with Stability to COX-2.ACS Med Chem Lett. 2024 May 28;15(6):965-971. doi: 10.1021/acsmedchemlett.4c00175. eCollection 2024 Jun 13. ACS Med Chem Lett. 2024. PMID: 38894922 Free PMC article.
-
Catalytic enantioselective silylation of acyclic and cyclic triols: application to total syntheses of cleroindicins D, F, and C.Angew Chem Int Ed Engl. 2009;48(3):547-50. doi: 10.1002/anie.200805338. Angew Chem Int Ed Engl. 2009. PMID: 19072962 Free PMC article. No abstract available.
-
Endocannabinoid binding to the cannabinoid receptors: what is known and what remains unknown.Curr Med Chem. 2010;17(14):1468-86. doi: 10.2174/092986710790980005. Curr Med Chem. 2010. PMID: 20166921 Free PMC article. Review.
-
Fatty acid amide signaling molecules.Bioorg Med Chem Lett. 2010 Oct 15;20(20):5959-68. doi: 10.1016/j.bmcl.2010.08.048. Epub 2010 Aug 13. Bioorg Med Chem Lett. 2010. PMID: 20817522 Free PMC article. Review.
-
A novel monoacylglycerol lipase inhibitor with analgesic and anti-inflammatory activity.Bioorg Med Chem Lett. 2008 Oct 15;18(20):5424-7. doi: 10.1016/j.bmcl.2008.09.039. Epub 2008 Sep 12. Bioorg Med Chem Lett. 2008. PMID: 18819796 Free PMC article.
References
-
- Mechoulam R, Ben-Shabat S, Hanuš L, Ligumsky M, Kaminski NE, Schatz AR, Gopher A, Almog S, Martin BR, Compton DR, Pertwee RG, Griffin G, Bayewitch M, Barg J, Vogel Z. Biochem Pharmacol. 1995;50:83. - PubMed
-
- Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, Yamashita A, Waku K. Biochem Biophys Res Commun. 1995;215:89. - PubMed
-
- Sugiura T, Kodaka T, Nakane S, Miyashita T, Kondo S, Suhara Y, Takayama H, Waku K, Seki C, Baba N, Ishima Y. J Biol Chem. 1999;274:2794. - PubMed
-
- Sugiura T, Kondo S, Kishimoto S, Miyashita T, Nakane S, Kodaka T, Suhara Y, Takayama H, Waku K. J Biol Chem. 2000;275:605. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information

