Fronto-temporal brain systems supporting spoken language comprehension
- PMID: 17827104
- PMCID: PMC2494576
- DOI: 10.1098/rstb.2007.2158
Fronto-temporal brain systems supporting spoken language comprehension
Abstract
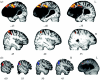
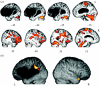
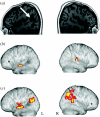
The research described here combines psycholinguistically well-motivated questions about different aspects of human language comprehension with behavioural and neuroimaging studies of normal performance, incorporating both subtractive analysis techniques and functional connectivity methods, and applying these tasks and techniques to the analysis of the functional and neural properties of brain-damaged patients with selective linguistic deficits in the relevant domains. The results of these investigations point to a set of partially dissociable sub-systems supporting three major aspects of spoken language comprehension, involving regular inflectional morphology, sentence-level syntactic analysis and sentence-level semantic interpretation. Differential patterns of fronto-temporal connectivity for these three domains confirm that the core aspects of language processing are carried out in a fronto-temporo-parietal language system which is modulated in different ways as a function of different linguistic processing requirements. No one region or sub-region holds the key to a specific language function; each requires the coordination of activity within a number of different regions. Functional connectivity analysis plays the critical role of indicating the regions which directly participate in a given sub-process, by virtue of their joint time-dependent activity. By revealing these codependencies, connectivity analysis sharpens the pattern of structure-function relations underlying specific aspects of language performance.
Figures





Similar articles
-
Lateralization of auditory language functions: a dynamic dual pathway model.Brain Lang. 2004 May;89(2):267-76. doi: 10.1016/S0093-934X(03)00351-1. Brain Lang. 2004. PMID: 15068909 Review.
-
Development of a selective left-hemispheric fronto-temporal network for processing syntactic complexity in language comprehension.Neuropsychologia. 2016 Mar;83:274-282. doi: 10.1016/j.neuropsychologia.2015.09.003. Epub 2015 Sep 6. Neuropsychologia. 2016. PMID: 26352468 Free PMC article.
-
Neural networks for sentence comprehension and production: An ALE-based meta-analysis of neuroimaging studies.Hum Brain Mapp. 2019 Jun 1;40(8):2275-2304. doi: 10.1002/hbm.24523. Epub 2019 Jan 28. Hum Brain Mapp. 2019. PMID: 30689268 Free PMC article.
-
The role of left inferior frontal and superior temporal cortex in sentence comprehension: localizing syntactic and semantic processes.Cereb Cortex. 2003 Feb;13(2):170-7. doi: 10.1093/cercor/13.2.170. Cereb Cortex. 2003. PMID: 12507948
-
Neuroimaging studies of language production and comprehension.Annu Rev Psychol. 2003;54:91-114. doi: 10.1146/annurev.psych.54.101601.145128. Epub 2002 Jun 10. Annu Rev Psychol. 2003. PMID: 12359916 Free PMC article. Review.
Cited by
-
Neural correlates of bridging inferences and coherence processing.J Psycholinguist Res. 2012 Aug;41(4):311-21. doi: 10.1007/s10936-011-9185-z. J Psycholinguist Res. 2012. PMID: 22113486
-
Non-invasive assessment of hemispheric language dominance by optical topography during a brief passive listening test: a pilot study.Med Sci Monit. 2011 Dec;17(12):CR692-7. doi: 10.12659/msm.882128. Med Sci Monit. 2011. PMID: 22129900 Free PMC article.
-
Age-Related Atrophy and Compensatory Neural Networks in Reading Comprehension.J Int Neuropsychol Soc. 2019 Jul;25(6):569-582. doi: 10.1017/S1355617719000274. Epub 2019 Apr 29. J Int Neuropsychol Soc. 2019. PMID: 31030698 Free PMC article.
-
Individual differences in verbal abilities associated with regional blurring of the left gray and white matter boundary.J Neurosci. 2011 Oct 26;31(43):15257-63. doi: 10.1523/JNEUROSCI.3039-11.2011. J Neurosci. 2011. PMID: 22031871 Free PMC article.
-
Written distractor words influence brain activity during overt picture naming.Front Hum Neurosci. 2014 Mar 24;8:167. doi: 10.3389/fnhum.2014.00167. eCollection 2014. Front Hum Neurosci. 2014. PMID: 24715859 Free PMC article.
References
-
- Barbas H. Anatomic basis of cognitive–emotional interactions in the primate prefrontal cortex. Neurosci. Biobehav. Rev. 1995;19:499–510. doi:10.1016/0149-7634(94)00053-4 - DOI - PubMed
-
- Basser P.J, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J. Magn. Reson. B. 1996;111:209–219. doi:10.1006/jmrb.1996.0086 - DOI - PubMed
-
- Beretta A, Campbell C, Carr T.H, Huang J, Schmitt L.M, Christianson K, Cao Y. An ER-fMRI investigation of morphological inflection in German reveals that the brain makes a distinction between regular and irregular forms. Brain Lang. 2003;85:67–92. doi:10.1016/S0093-934X(02)00560-6 - DOI - PubMed
-
- Binder J.R, Frost J.A, Hammeke T.A, Bellgowan P.S.F, Springer J.A, Kaufman J.N. Human temporal lobe activation by speech and nonspeech sounds. Cereb. Cortex. 2000;10:512–528. doi:10.1093/cercor/10.5.512 - DOI - PubMed
-
- Bokde A.L.W, Tagamets M.-A, Friedman R.B, Horwitz B. Functional interactions of the inferior frontal cortex during the processing of words and word-like stimuli. Neuron. 2001;30:609–617. doi:10.1016/S0896-6273(01)00288-4 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

