Tip60 functions as a potential corepressor of KLF4 in regulation of HDC promoter activity
- PMID: 17827213
- PMCID: PMC2094084
- DOI: 10.1093/nar/gkm656
Tip60 functions as a potential corepressor of KLF4 in regulation of HDC promoter activity
Abstract
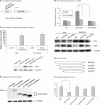
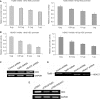
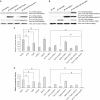
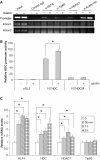
KLF4 is a transcription factor that is highly expressed in the gastrointestinal tract. Previously we have demonstrated that KLF4 represses HDC promoter activity in a gastric cell line through both an upstream Sp1 binding GC box and downstream gastrin responsive elements. However, the mechanism by which KLF4 inhibits HDC promoter is not well defined. In the current study, by using yeast two-hybrid screening, Tip60 was identified as a KLF4 interacting protein. Further coimmunoprecipitation and functional reporter assays support the interaction between these two proteins. In addition, Tip60 and HDAC7, previously shown to interact with each other and repress transcription, inhibited HDC promoter activity in a dose-dependent fashion. Consistently, knock down of Tip60 or HDAC7 gene expression by specific shRNA increased endogenous HDC mRNA level. Co-immunoprecipitation assays showed that HDAC7 was pulled down by KLF4 and Tip60, suggesting that these three proteins form a repressive complex. Further chromatin immuno-precipitation indicated that all three proteins associated with HDC promoter. Two-hour gastrin treatment, known to activate HDC gene expression, significantly decreased the association of KLF4, Tip60 and HDAC7 with HDC promoter, suggesting that gastrin activates HDC gene expression at least partly by decreasing the formation of KLF4/Tip60/HDAC7 repressive complexes at the HDC promoter.
Figures




References
-
- Garrett-Sinha LA, Eberspaecher H, Seldin MF, de Crombrugghe B. A gene for a novel zinc-finger protein expressed in differentiated epithelial cells and transiently in certain mesenchymal cells. J. Biol. Chem. 1996;271:31384–31390. - PubMed
-
- Yet SF, McA'Nulty MM, Folta SC, Yen HW, Yoshizumi M, Hsieh CM, Layne MD, Chin MT, Wang H, et al. Human EZF, a Kruppel-like zinc finger protein, is expressed in vascular endothelial cells and contains transcriptional activation and repression domains. J. Biol. Chem. 1998;273:1026–1031. - PubMed
-
- Black AR, Black JD, Azizkhan-Clifford J. Sp1 and kruppel-like factor family of transcription factors in cell growth regulation and cancer. J. Cell Physiol. 2001;188:143–160. - PubMed
-
- Chen ZY, Shie JL, Tseng CC. Gut-enriched Kruppel-like factor represses ornithine decarboxylase gene expression and functions as checkpoint regulator in colonic cancer cells. J. Biol. Chem. 2002;277:46831–46839. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

