The role of phosphoenolpyruvate carboxylase during C4 photosynthetic isotope exchange and stomatal conductance
- PMID: 17827274
- PMCID: PMC2048775
- DOI: 10.1104/pp.107.103390
The role of phosphoenolpyruvate carboxylase during C4 photosynthetic isotope exchange and stomatal conductance
Abstract
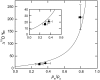
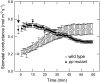
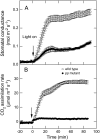
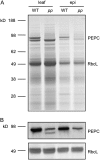
Phosphoenolpyruvate carboxylase (PEPC; EC 4.1.1.31) plays a key role during C(4) photosynthesis and is involved in anaplerotic metabolism, pH regulation, and stomatal opening. Heterozygous (Pp) and homozygous (pp) forms of a PEPC-deficient mutant of the C(4) dicot Amaranthus edulis were used to study the effect of reduced PEPC activity on CO(2) assimilation rates, stomatal conductance, and (13)CO(2) (Delta(13)C) and C(18)OO (Delta(18)O) isotope discrimination during leaf gas exchange. PEPC activity was reduced to 42% and 3% and the rates of CO(2) assimilation in air dropped to 78% and 10% of the wild-type values in the Pp and pp mutants, respectively. Stomatal conductance in air (531 mubar CO(2)) was similar in the wild-type and Pp mutant but the pp mutant had only 41% of the wild-type steady-state conductance under white light and the stomata opened more slowly in response to increased light or reduced CO(2) partial pressure, suggesting that the C(4) PEPC isoform plays an essential role in stomatal opening. There was little difference in Delta(13)C between the Pp mutant (3.0 per thousand +/- 0.4 per thousand) and wild type (3.3 per thousand +/- 0.4 per thousand), indicating that leakiness (), the ratio of CO(2) leak rate out of the bundle sheath to the rate of CO(2) supply by the C(4) cycle, a measure of the coordination of C(4) photosynthesis, was not affected by a 60% reduction in PEPC activity. In the pp mutant Delta(13)C was 16 per thousand +/- 3.2 per thousand, indicative of direct CO(2) fixation by Rubisco in the bundle sheath at ambient CO(2) partial pressure. Delta(18)O measurements indicated that the extent of isotopic equilibrium between leaf water and the CO(2) at the site of oxygen exchange () was low (0.6) in the wild-type and Pp mutant but increased to 0.9 in the pp mutant. We conclude that in vitro carbonic anhydrase activity overestimated as compared to values determined from Delta(18)O in wild-type plants.
Figures


References
-
- Allaway WG (1973) Accumulation of malate in guard cells of Vicia faba during stomatal opening. Planta 110 63–70 - PubMed
-
- Andreo CS, Gonzales D, Inlesias A (1987) Higher plant phosphoenolpyruvate carboxylase: structure and regulation. FEBS Lett 213 1–8
-
- Asai N, Nakajima N, Tamaoki M, Kamada H, Kondo N (2000) Role of malate synthesis mediated by phosphoenolpyruvate carboxylase in guard cells in the regulation of stomatal movement. Plant Cell Physiol 41 10–15 - PubMed
-
- Britto DT, Kronzucker HJ (2005) Nitrogen acquisition, PEP carboxylase, and cellular pH homeostasis: new news on old paradigms. Plant Cell Environ 28 1396–1409
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

