Induction of immune responses in mice after intragastric administration of Lactobacillus casei producing porcine parvovirus VP2 protein
- PMID: 17827311
- PMCID: PMC2074969
- DOI: 10.1128/AEM.00436-07
Induction of immune responses in mice after intragastric administration of Lactobacillus casei producing porcine parvovirus VP2 protein
Abstract
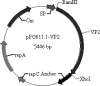
Lactobacillus casei ATCC 393 was selected as an antigen delivery vehicle for mucosal immunization against porcine parvovirus (PPV) infection. A 64-kDa fragment of PPV major protective antigen VP2 protein was used as the parvovirus antigen model. A recombinant Lactobacillus expressing VP2 protein was constructed with plasmid pPG611.1, where expression and localization of the VP2 protein from recombinant Lc393-rPPV-VP2 was detected via sodium dodecyl sulfate-polyacrylamide gel electrophoresis, Western blotting, and immunofluorescence. Both local mucosal and systemic immune responses against PPV were induced in BALB/c mice immunized orally with the recombinant Lactobacillus expressing VP2 protein. The induced antibodies demonstrated neutralizing effects on PPV infection. These data indicated that the use of recombinant lactobacilli could be a valuable strategy for future vaccine development of PPV.
Figures







Similar articles
-
Comparison of the immune responses induced by oral immunization of mice with Lactobacillus casei-expressing porcine parvovirus VP2 and VP2 fused to Escherichia coli heat-labile enterotoxin B subunit protein.Comp Immunol Microbiol Infect Dis. 2011 Jan;34(1):73-81. doi: 10.1016/j.cimid.2010.02.004. Epub 2010 Mar 11. Comp Immunol Microbiol Infect Dis. 2011. PMID: 20226529 Free PMC article.
-
Construction of recombinant Lactobacillus casei efficiently surface displayed and secreted porcine parvovirus VP2 protein and comparison of the immune responses induced by oral immunization.Immunology. 2008 May;124(1):68-75. doi: 10.1111/j.1365-2567.2007.02738.x. Epub 2007 Nov 22. Immunology. 2008. PMID: 18034821 Free PMC article.
-
[Co-expression of CSFV T cell epitope E290 peptide and PPV VP2 protein in Lactobacillus casei and determination of specific antibodies in immunized mice].Wei Sheng Wu Xue Bao. 2007 Aug;47(4):667-72. Wei Sheng Wu Xue Bao. 2007. PMID: 17944369 Chinese.
-
[The surface display of porcine parvovirus VP2 protein in Lactobacillus casei].Sheng Wu Gong Cheng Xue Bao. 2007 Mar;23(2):315-8. Sheng Wu Gong Cheng Xue Bao. 2007. PMID: 17460908 Chinese.
-
[Protection against Salmonella via immunization with recombinant lactic acid bacteria].Nihon Rinsho. 2012 Aug;70(8):1293-7. Nihon Rinsho. 2012. PMID: 22894061 Review. Japanese.
Cited by
-
Oral Immunization of Chickens With Recombinant Lactobacillus plantarum Vaccine Against Early ALV-J Infection.Front Immunol. 2019 Oct 2;10:2299. doi: 10.3389/fimmu.2019.02299. eCollection 2019. Front Immunol. 2019. PMID: 31632395 Free PMC article.
-
An EGFP-marked recombinant lactobacillus oral tetravalent vaccine constitutively expressing α, ε, β1, and β2 toxoids for Clostridium perfringens elicits effective anti-toxins protective immunity.Virulence. 2019 Dec;10(1):754-767. doi: 10.1080/21505594.2019.1653720. Virulence. 2019. PMID: 31429624 Free PMC article.
-
Strategy of Developing Oral Vaccine Candidates Against Co-infection of Porcine Diarrhea Viruses Based on a Lactobacillus Delivery System.Front Microbiol. 2022 Apr 4;13:872550. doi: 10.3389/fmicb.2022.872550. eCollection 2022. Front Microbiol. 2022. PMID: 35444630 Free PMC article.
-
Immunogenicity of recombinant classic swine fever virus CD8(+) T lymphocyte epitope and porcine parvovirus VP2 antigen coexpressed by Lactobacillus casei in swine via oral vaccination.Clin Vaccine Immunol. 2011 Nov;18(11):1979-86. doi: 10.1128/CVI.05204-11. Epub 2011 Sep 21. Clin Vaccine Immunol. 2011. PMID: 21940406 Free PMC article.
-
Isolation and characterization of porcine parvovirus in Vietnam.Vet World. 2024 Jul;17(7):1530-1537. doi: 10.14202/vetworld.2024.1530-1537. Epub 2024 Jul 13. Vet World. 2024. PMID: 39185042 Free PMC article.
References
-
- Brandtzaeg, P. 1994. Distribution and characteristics of mucosal immunoglobulin-producing cells, p. 251-279. In O. L. Pearay and H. Metecky (ed.), Handbook of mucosal immunology. Academic Press, Boston, MA.
-
- Carwright, S. F., and R. A. Huck. 1967. Viruses isolated in association with herd infertility, abortions and stillbirths in pigs. Vet. Rec. 81:196-197.
-
- Challacombe, S. J. 1983. Salivary antibodies and systemic tolerance in mice after oral immunization with bacterial antigens. Ann. N. Y. Acad. Sci. 409:177-193. - PubMed
-
- Chang, Y. H., J. K. Kim, and H. J. Kim. 2001. Selection of a potential probiotic Lactobacillus strain and subsequent in vivo studies. Antonie Leeuwenhoek 80:193-199. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

