Aberrant endometrial features of pregnancy in diabetic NOD mice
- PMID: 17827401
- PMCID: PMC2949414
- DOI: 10.2337/db07-0773
Aberrant endometrial features of pregnancy in diabetic NOD mice
Abstract
Objective: Pregnant diabetic women are at a 4-12 times higher risk for preeclampsia, an urgent acute-onset complication of mid- to late gestation, than normal pregnant women. Hallmarks of preeclampsia are hypertension, proteinuria, and incomplete modification of endometrial spiral arteries. Transient proangiogenic lymphocytes called uterine natural killer (uNK) cells are implicated in human and rodent spiral artery modification. We studied mid- to late gestations in spontaneously type 1 diabetic NOD mice to investigate whether diabetes alters uNK cell homing and/or function.
Research design and methods: Normoglycemic, pre-diabetic, and diabetic NOD mice and controls were mated. Lymphocytes and endometrial endothelium and decidua were studied histologically and in functional assays.
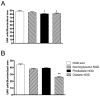
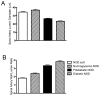
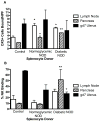
Results: Conception accelerated progression to overt diabetes in NOD females who had limited spiral artery development, heavier placentas, and lighter fetuses displaying numerous birth defects compared with controls. UNK cell numbers were reduced in the decidua basalis of diabetic females, whereas interferon-gamma production was elevated. In diabetic NOD mice, decidual expression of the mucosal vascular addressin cell adhesion molecule (MAdCAM)-1 was aberrant in position, whereas vascular cell adhesion molecule (VCAM)-1 expression was reduced. Assays of lymphocyte adhesion to tissue sections under shear forces indicated that diabetes compromises the potential homing functions of both endometrial endothelium and peripheral NK cells.
Conclusions: In diabetes, gestational endometrium has immune and vascular defects that likely contribute to murine fetal loss and birth defects. Analogous problems and preeclampsia in diabetic women may involve similar mechanisms.
Figures

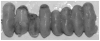




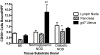
References
-
- Leiter EH. The NOD mouse: a model for insulin-dependent diabetes mellitis. Current Protocols in Immunology. 1997;24(suppl):15.9.1–15.9.23. - PubMed
-
- Wicker LS, Clark J, Fraser HI, Garner VE, Gonzalez-Munoz A, Healy B, Howlett S, Hunter K, Rainbow D, Rosa RL, Smink LJ, Todd JA, Peterson LB. Type 1 diabetes genes and pathways shared by humans and NOD mice. J Autoimmun. 2005;25 (Suppl):29–33. - PubMed
-
- Wicker LS, Todd JA, Peterson LB. Genetic control of autoimmune diabetes in the NOD mouse. Annu Rev Immunol. 1995;13:179–200. - PubMed
-
- Serreze DV, Leiter EH. Defective activation of T suppressor cell function in nonobese diabetic mice. Potential relation to cytokine deficiencies. J Immunol. 1988;140:3801–3807. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

